Ren1
-
Official Full Name
Renin 1 Structural -
Overview
Renin catalyzes the first step in the activation pathway of angiotensinogen--a cascade that can result in aldosterone release, vasoconstriction, and increase in blood pressure. Renin, an aspartyl protease, cleaves angiotensinogen to form angiotensin I, which is converted to angiotensin II by angiotensin I converting enzyme, an important regulator of blood pressure and electrolyte balance. Transcript variants that encode different protein isoforms and that arise from alternative splicing and the use of alternative promoters have been described, but their full-length nature has not been determined. Mutations in this gene have been shown to cause familial hyperproreninemia. -
Synonyms
Ren1;renin 1 structural;Ren;Rnr;Rn-1;Ren-1;Ren-A;Ren1c;Ren1d;D19352;renin-1;renin b;kidney renin;aspartyl-protease;OTTMUSP00000024128;angiotensinogenase;renin;EC 3.4.23.15;renin precursor, renal;HNFJ2;FLJ107612;EC 3.4.238;angiotensin-forming enzyme;Prorenin;FLJ10761;RENAA;Renin precursor renal;Angiotensin forming enzyme
Recombinant Proteins
- Mouse
- HEK293
- Mammalian Cells
- Insect Cells
- E.coli
- Human Cells
- His
- Non
- DDK
- Myc
- Avi
- Fc
| Cat.# | Product name | Source (Host) | Species | Tag | Protein Length | Price |
|---|---|---|---|---|---|---|
| Ren1-2305M | Active Recombinant Mouse Ren1 protein, His-tagged | HEK293 | Mouse | His | 1-402 a.a. | |
| REN1-14072M | Recombinant Mouse REN1 Protein | Mammalian Cells | Mouse | His |
|
|
| Ren1-379M | Recombinant Mouse Ren1 Protein, His-tagged | Insect Cells | Mouse | His |
|
|
| Ren1-7885M | Recombinant Mouse Ren1 protein, His-tagged | E.coli | Mouse | His | Val110~Val221 |
|
| Ren1-7886M | Recombinant Mouse Ren1 protein, His-tagged | E.coli | Mouse | His | Thr265~Tyr386 |
|
| REN-2576MCL | Recombinant Mouse REN cell lysate | Human Cells | Mouse | Non |
|
|
| Ren1-5461M | Recombinant Mouse Ren1 Protein, Myc/DDK-tagged | HEK293 | Mouse | DDK&Myc |
|
|
| Ren1-7315M | Recombinant Mouse Ren1 Protein, His-tagged | Insect Cells | Mouse | His | 22-402 |
|
| REN1-7522M | Recombinant Mouse REN1 Protein, His (Fc)-Avi-tagged | HEK293 | Mouse | Avi&Fc&His |
|
|
| REN1-7522M-B | Recombinant Mouse REN1 Protein Pre-coupled Magnetic Beads | HEK293 | Mouse |
|
||
| REN1-809M | Recombinant Mouse REN1 Protein | HEK293 | Mouse | 402 |
|
Background
What is REN1 protein?
REN1 (renin 1 structural) gene is a protein coding gene which is orthologous to human REN (renin). It Enables endopeptidase activity. Acts upstream of or within with a positive effect on regulation of blood pressure. Acts upstream of or within several processes, including drinking behavior; male gonad development; and regulation of systemic arterial blood pressure by renin-angiotensin. Located in extracellular space. Is expressed in several structures, including alimentary system; brain; extraembryonic component; genitourinary system; and trunk. REN1 protein is consisted of 402 amino acids and its molecular mass is approximately 44.3 kDa.
What is the function of REN1 protein?
The REN1 protein, or renin, is a highly specific endopeptidase whose primary function is to produce angiotensin I from angiotensinogen in the plasma, a process that initiates a chain of reactions that lead to increased blood pressure and increased sodium ion retention in the kidneys. Renin activity is regulated by a variety of factors, including interactions with ATP6AP2, which can increase renin's efficiency in treating angiotensinogen. Renin is synthesized by the para-bulbar cells of the kidneys in response to decreased blood pressure and sodium concentrations. In addition, the renin-angiotensin system (RAS) plays a central role in the regulation of blood pressure and fluid electrolyte balance. The expression and activity of renin is essential for the maintenance of homeostasis, and its abnormal regulation may lead to cardiovascular diseases such as hypertension.
REN1 Related Signaling Pathway
Renin's main function is to convert angiotensinogen to angiotensin I, a key step in RAS. RAS plays a central role in regulating blood pressure and fluid electrolyte balance. REN1 regulates blood pressure through RAS, affecting drinking behavior, male gonad development, and systemic arterial blood pressure regulation. Renin regulation is also influenced by the cAMP signaling pathway, one of the three major signaling pathways that regulate renin expression and activity. Notch signaling pathway is also involved in regulating renin expression and activity. Renin expression is also influenced by activation of the glucagon-like peptide-1 receptor (GLP-1R), which induces the expression of the REN1 gene.
REN1 Related Diseases
Renin encoded by the REN1 gene is a key component of the renin-angiotensin-aldosterone system (RAAS), which plays an important role in blood pressure regulation. Abnormally elevated renin levels can lead to high blood pressure. Although HFMD is primarily associated with enterovirus infection, in some cases, variations in the REN1 gene may be associated with the severity of the disease. Renin expressed by REN1 gene plays a role in renal function, and its abnormality may be related to the occurrence and development of various renal diseases, such as renal artery stenosis and renal tumor. Portal hypertension is a liver disease associated with blocked blood flow in the liver. The REN1 gene is related to angiotensin production, which may indirectly affect the development of portal hypertension.
Case Study
Case Study 1: Hirofumi Watanabe, 2021
Renin-expressing cells are myoendocrine cells crucial for survival. They have been postulated to possess a pressure-sensing mechanism, a baroreceptor, that can detect slight changes in blood pressure and respond with precise and synchronized amounts of renin synthesized and released to the circulation to maintain blood pressure and fluid-electrolyte balance. The location and nature of this puzzling pressure-sensing structure have remained unknown since it was originally suggested over sixty years ago. This study aims to elucidate the location and structure of the renin cell baroreceptor. The researchers used a variety of genetically modified mice whereby renin cells were exposed in vivo to either low or high arterial pressure. In addition, they applied direct mechanical stimuli, i.e., pneumatic pressure or stretch, directly to renin cells cultured under different conditions and substrata. Changes in perfusion pressure and/or direct mechanical stimuli induced significant changes in renin gene expression and the phenotype of renin cells. Importantly, the experiments show that the pressure-sensing mechanism (the baroreceptor) resides in the renin cells; it requires initial extracellular sensing by integrin β1 at the renin cell membrane and is transduced to the nuclear membrane and chromatin by lamin A/C.
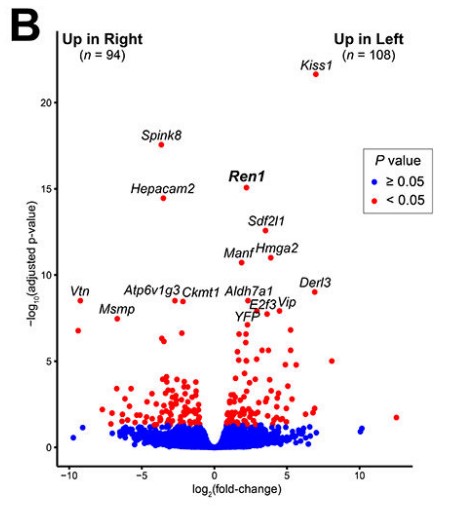
Fig1. Volcano plot of RNA-seq analysis.
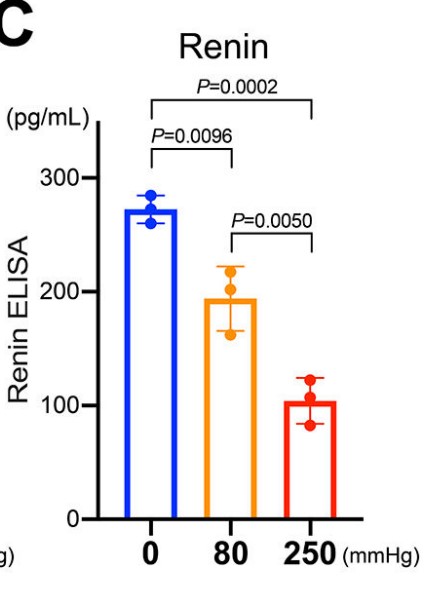
Fig2. ELISA for renin in the culture medium showed that secretion of renin protein from renin cells also decreased in response to the pneumatic pressure.
Case Study 2: Wei Chen, 2018
As a universal energy generation pathway utilizing carbon metabolism, glycolysis plays an important housekeeping role in all organisms. Pollen tubes expand rapidly via a mechanism of polarized growth, known as tip growth, to deliver sperm for fertilization. Here, the researchers report a novel and surprising role of glycolysis in the regulation of growth polarity in Arabidopsis pollen tubes via impingement of Rho GTPase-dependent signaling. They identified a cytosolic phosphoglycerate kinase (pgkc-1) mutant with accelerated pollen germination and compromised pollen tube growth polarity. pgkc-1 mutation greatly diminished apical exocytic vesicular distribution of REN1 RopGAP (Rop GTPase activating protein), leading to ROP1 hyper-activation at the apical plasma membrane. Consequently, pgkc-1 pollen tubes contained higher amounts of exocytic vesicles and actin microfilaments in the apical region, and showed reduced sensitivity to Brefeldin A and Latrunculin B, respectively. While inhibition of mitochondrial respiration could not explain the pgkc-1 phenotype, the glycolytic activity is indeed required for PGKc function in pollen tubes. Moreover, the pgkc-1 pollen tube phenotype was mimicked by the inhibition of another glycolytic enzyme.

Fig3. GFP-REN1 localization in WT and pgkc-1 pollen tubes.

Fig4. Pollen tube morphology ren1-3 plants subjected to mock treatment.
Quality Guarantee
High Purity
.jpg)
Fig1. SDS-PAGE (Ren1-7315M)
Involved Pathway
Ren1 involved in several pathways and played different roles in them. We selected most pathways Ren1 participated on our site, such as Renin-angiotensin system,Renin secretion, which may be useful for your reference. Also, other proteins which involved in the same pathway with Ren1 were listed below. Creative BioMart supplied nearly all the proteins listed, you can search them on our site.
| Pathway Name | Pathway Related Protein |
|---|---|
| Renin-angiotensin system | KLK1B11,KLK1B24,AGTR1A,AGTR1B,REN,KLK1B5,LNPEP,REN2,PREP,CPA3 |
| Renin secretion | CALML3,PPP3R1,PLCB1,CLCA4,PPP3CC,GNAQ,PRKACG,GNAI3,PLCB2,PDE1C |
Protein Function
Ren1 has several biochemical functions, for example, aspartic-type endopeptidase activity,endopeptidase activity,hydrolase activity. Some of the functions are cooperated with other proteins, some of the functions could acted by Ren1 itself. We selected most functions Ren1 had, and list some proteins which have the same functions with Ren1. You can find most of the proteins on our site.
| Function | Related Protein |
|---|---|
| peptidase activity | HPN,FAM105B,PROCA,TMPRSS12,CTSG,C1rb,APH1C,ASTL,Adamts19,CAPN2B |
| hydrolase activity | CPSF3L,Aadacl3,ADAMTS16,NUDT13,PTPRK,THSD4,PPM1BA,OTUD4,INPPL1,CTSLA |
| endopeptidase activity | MME,PCSK6,FAP,KLK1B11,TPP1,PSMB9B,KLK1B9,HTRA4,PSEN2,KLK1B24 |
| insulin-like growth factor receptor binding | INS,ARRB1,SOCS2,SOCS1,REN,IGF2A,GNAS,INSL4,INS2,YWHAH |
| aspartic-type endopeptidase activity | PGA,DDI2,REN2,BACE2,PSEN2,NRIP3,PSEN1,PGC,NRIP2,ANK2B |
| receptor binding | FYB,CROT,PLA2G1B,LRRC4B,NRG2B,PIPOX,PTPRD,FGF1B,FGF18B,WNT2BB |
Interacting Protein
Ren1 has direct interactions with proteins and molecules. Those interactions were detected by several methods such as yeast two hybrid, co-IP, pull-down and so on. We selected proteins and molecules interacted with Ren1 here. Most of them are supplied by our site. Hope this information will be useful for your research of Ren1.
Ywhae
Resources
Related Services
Related Products
References
- Cuffe, JSM; Walton, SL; et al. The effects of gestational age and maternal hypoxia on the placental renin angiotensin system in the mouse. PLACENTA 35:953-961(2014).
- Xa, LK; Lacombe, MJ; et al. General lysosomal hydrolysis can process prorenin accurately. AMERICAN JOURNAL OF PHYSIOLOGY-REGULATORY INTEGRATIVE AND COMPARATIVE PHYSIOLOGY 307:R505-R513(2014).


