SUMO1
-
Official Full Name
small ubiquitin-like modifier 1 -
Overview
This gene encodes a protein that is a member of the SUMO (small ubiquitin-like modifier) protein family. It functions in a manner similar to ubiquitin in that it is bound to target proteins as part of a post-translational modification system. However, unlike ubiquitin which targets proteins for degradation, this protein is involved in a variety of cellular processes, such as nuclear transport, transcriptional regulation, apoptosis, and protein stability. It is not active until the last four amino acids of the carboxy-terminus have been cleaved off. Several pseudogenes have been reported for this gene. Alternate transcriptional splice variants encoding different isoforms have been characterized. [provided by RefSeq, Jul 2008] -
Synonyms
SUMO1;small ubiquitin-like modifier 1;DAP1;GMP1;PIC1;SMT3;UBL1;OFC10;SENP2;SMT3C;SMT3H3;small ubiquitin-related modifier 1;sentrin;SMT3 homolog 3;GAP modifying protein 1;ubiquitin-like protein UBL1;ubiquitin-like protein SMT3C;SMT3 suppressor of mif two 3 homolog 1;ubiquitin-homology domain protein PIC1
Recombinant Proteins
- Human
- Zebrafish
- Mouse
- Rhesus macaque
- Chicken
- Rat
- E. coli
- E.coli
- Mammalian Cell
- Human
- HEK293
- GST
- His
- Non
- HA
- His&Fc&Avi
Background

Fig1. Potential mechanisms of SUMO1 in different heart cell types after myocardial infarction (MI). (Zhihao Liu, 2023)
What is SUMO1 protein?
SUMO1 (small ubiquitin like modifier 1) gene is a protein coding gene which situated on the long arm of chromosome 2 at locus 2q33. This gene encodes a protein that is a member of the SUMO (small ubiquitin-like modifier) protein family. It functions in a manner similar to ubiquitin in that it is bound to target proteins as part of a post-translational modification system. However, unlike ubiquitin which targets proteins for degradation, this protein is involved in a variety of cellular processes, such as nuclear transport, transcriptional regulation, apoptosis, and protein stability. It is not active until the last four amino acids of the carboxy-terminus have been cleaved off. The SUMO1 protein is consisted of 101 amino acids and its molecular mass is approximately 11.6 kDa.
What is the function of SUMO1 protein?
SUMO1 protein is a small ubiquitin related modifier, SUMO1 can affect the transport of proteins between the nucleus and cytoplasm, thereby regulating the expression of specific genes. By modifying proteins involved in DNA repair and replication, SUMO1 helps maintain the stability of the genome. It can change the function of transcription factors and chromatin structure regulatory proteins, and then affect the transcriptional activity of genes. By interacting with other proteins, SUMO1 can influence the stability and degradation rate of proteins. When cells are subjected to various stressful conditions (such as heat shock, oxidative stress, etc.), the expression and modification activity of SUMO1 changes to help cells adapt to and respond to these stresses.
SUMO1 Related Signaling Pathway
SUMO1 can modify certain nuclear hormone receptors such as estrogen receptors, affecting their transcriptional activity and downstream gene expression. SUMO1 modification is related to the recognition and repair process of DNA double-strand breaks and affects the repair efficiency by acting on the components of the repair complex. By interacting with the ubiquitin system, SUMO1 can sometimes influence the ubiquitination state of a target protein, thereby altering its degradation rate in the proteasome pathway. The influences include cell cycle regulation, apoptosis, transcription factor regulation and so on.
SUMO1 Related Diseases
Abnormal SUMO modification is associated with a variety of neurodegenerative diseases, such as Alzheimer's disease, Huntington's disease, and Parkinson's disease. Studies have found that SUMO1 plays an important role in heart development and the occurrence of heart disease. Some viruses, such as human immunodeficiency virus (HIV) and influenza virus, use the SUMO system of host cells to facilitate their replication and spread. SUMO1 also plays a role in muscle development and maintenance, and its abnormalities may be associated with muscle diseases such as muscular dystrophy. Several tumors and metabolic diseases are also associated with SUMO1.
Bioapplications of SUMO1
SUMO1 is involved in regulating multiple pathways that influence cell proliferation, apoptosis and tumor formation. Therefore, studying the role of SUMO1 in the development of cancer can help to discover new diagnostic markers and potential therapeutic targets. Due to the critical role of SUMO1 in many diseases, the development of agents capable of manipulating SUMO has become a direction of new drug development, especially in the treatment of cancer and neurodegenerative diseases.
Case Study
Case Study 1: Andreia Lee, 2019
Conjugation of small ubiquitin-like modifiers (SUMOs) to substrate proteins is a posttranslational protein modification that affects a diverse range of physiological processes. Global inhibition of SUMO conjugation in mice results in embryonic lethality, reflecting the importance of the SUMO pathways for embryonic development. Here, the researchers demonstrated that SUMO1 overexpression was not well tolerated in murine embryonic carcinoma and embryonic stem (ES) cells and that only a few clones were recovered after transduction with vectors delivering SUMO1 expression constructs. Differentiated NIH/3T3 cells overexpress SUMO1 without deleterious effects and maintain high levels of both conjugated and free forms of SUMO1. The few embryonic cells surviving after forced overexpression retained all their SUMO1 in the form of a few high-molecular-weight conjugates and maintained undetectable levels of free SUMO1. The absence of free SUMO in embryonic cells was seen specifically upon overexpression of SUMO1, but not SUMO2. Moreover, blocking SUMO1 conjugation to endogenous substrates by C-terminal mutations of SUMO1 or by overexpression of a SUMO1 substrate "sponge" or by overexpression of the deSUMOylating enzyme SUMO-specific peptidase 1 (SENP1) dramatically restored free SUMO1 overexpression.

Fig1. Western blot of lysates of embryonic cell lines (F9, PCC4, and E14 cells) transduced with vectors expressing either Flag-SUMO1 or Flag-SUMO1ΔGG.

Case Study 2: Changkang Ke, 2019
One previous study showed that SUMO1 expression is closely related to progression in non-small cell lung cancer (NSCLC); however, the function of SUMO1 in NSCLC has not yet been well elucidated. SUMO1 was enhanced or silenced in two NSCLC cell lines by using either forced SUMO1 expression or short hairpin RNA against SUMO1 lentiviral vectors, respectively. The biological functions of SUMO1 in NSCLC were investigated through colony-formation, cell proliferation, and invasion assays, and cell cycle analysis. NF-κB expression was detected in the overexpressed and silenced SUMO1 cell lines. Immunohistochemistry was used to detect an association between SUMO1 and NF-κB in the cancer and adjacent tissues of 168 patients with lung cancer. The results showed overexpressed SUMO1 promoted the proliferation rate, colony formation ability, invasion, and NF-κB expression in an A549 cell line. Conversely, SUMO1 depletion inhibited the cell growth rate, colony formation ability, invasion, and NF-κB expression in a Calu-1 cell line.

Fig3. Similar results were obtained through Western blot analysis.

Quality Guarantee
High Purity

Fig1. SDS-PAGE (SUMO1-7040H) (PROTOCOL for western blot)
.

Fig2. SDS-PAGE (SUMO1-1247H) (PROTOCOL for western blot)
Involved Pathway
SUMO1 involved in several pathways and played different roles in them. We selected most pathways SUMO1 participated on our site, such as RNA transport, which may be useful for your reference. Also, other proteins which involved in the same pathway with SUMO1 were listed below. Creative BioMart supplied nearly all the proteins listed, you can search them on our site.
| Pathway Name | Pathway Related Protein |
|---|---|
| RNA transport | NUP85,NUP153,EIF3C,THOC2,EIF1AY,EIF4E2RS1,NUP155,TACC3,SMN2,NUP62L |

Fig1. Schematic diagram of the effect and molecular mechanism of Smad2 SUMOylation in the progression of TGF-β-induced EndoMT. (Qi Su, 2023)

Fig2. Model of the effects of SUMO1 on the Wnt/β-Catenin signaling pathway in relation to colitis. (Jingyan Wang, 2020)
Protein Function
SUMO1 has several biochemical functions, for example, SUMO transferase activity,ion channel binding,poly(A) RNA binding. Some of the functions are cooperated with other proteins, some of the functions could acted by SUMO1 itself. We selected most functions SUMO1 had, and list some proteins which have the same functions with SUMO1. You can find most of the proteins on our site.
| Function | Related Protein |
|---|---|
| ion channel binding | CTNNB1,ANK2,KCNIP2,ACTN3,KCNH5,FXYD1,SRC,SCN3B,FGF13,RIMS1 |
| ubiquitin protein ligase binding | PER1,CKB,LYN,CUL1,H2-AB1,RAD18,FBXW7,CUL2,ATXN3,AMBRA1 |
| protein tag | SUMO4,SUMO2B,RNF41,UBL5,SUMO3A,ISG15,SUMO3,SUMO2,SUMO3B,NFATC2IP |
| poly(A) RNA binding | NSBP1,PWP2,DNAJC2,ADARB1,PRPF31,PYDC3,NCBP2,DDX55,LAS1L,UBE2N |
| potassium channel regulator activity | KCNIP4,KCNMB1,CHP,SGK2B,SGK2,ABCC9,KCNIP3,PRKCZ,DRD4,ARPP19A |
| protein binding | PRKDC,THAP10,DTX3L,RAD51B,NOS1AP,FCRL2,TUFT1,TMEM100,TAG-260,TMEM106B |
| transcription factor binding | WWP2,MDFI,GNPTAB,HDAC11,FOXF2,YWHAZ,HMGB2,TAF1,NFIA,RBL1 |
| SUMO transferase activity | SUMO3,SUMO4,UBE2IA,CBX4,PIAS3,TOPORS,UBE2IB,PIAS4,MUL1,UBE2I |
Interacting Protein
SUMO1 has direct interactions with proteins and molecules. Those interactions were detected by several methods such as yeast two hybrid, co-IP, pull-down and so on. We selected proteins and molecules interacted with SUMO1 here. Most of them are supplied by our site. Hope this information will be useful for your research of SUMO1.
UBE2I;DAXX;SP100
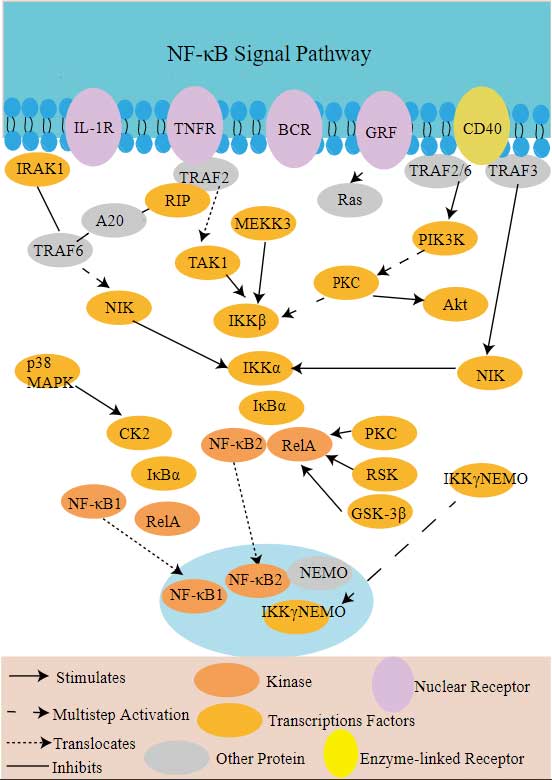
SUMO1 Related Signal Pathway
Resources
Research Area
Ubiquitin-like Modifiers (UBLs) and RegulatorsPost-translational Modification of NFkB/IkB
Related Services
Related Products
References
- Collaud, S; Tischler, V; et al. Lung neuroendocrine tumors: correlation of ubiquitinylation and sumoylation with nucleo-cytosolic partitioning of PTEN. BMC CANCER 15:-(2015).
- Barysch, SV; Dittner, C; et al. Identification and analysis of endogenous SUMO1 and SUMO2/3 targets in mammalian cells and tissues using monoclonal antibodies. NATURE PROTOCOLS 9:896-909(2014).