EGF Receptor
Related Symbol Search List
Immunology Background
Available Resources for EGF Receptor Research
Creative BioMart offers comprehensive support for researchers studying the EGF receptors, including a variety of carefully developed products, customized services to meet specific research needs and a wide range of resources. These valuable resources provide researchers with an invaluable reference for deepening their understanding of the EGF receptor molecules and their critical role in various physiological processes.
- Our product range includes recombinant proteins, protein pre-coupled magnetic beads, cell and tissue lysates, and more.
- We are also dedicated to providing a wealth of resources on EGF receptor molecules, with resources covering important topics including involved pathways, protein functions, interacting proteins, related articles, and research areas.
Our Featured Products
| Cat.# | Product name | Species | Source (Host) | Tag |
|---|---|---|---|---|
| EGFR-692HA | Active Recombinant Human EGFR protein, Fc-tagged, APC labeled | Human | HEK293 | Fc |
| ERBB2-033H | Active Recombinant Human ERBB2 Protein, Fc-tagged | Human | HEK293 | Fc |
| ERBB3-383H | Recombinant Human ERBB3 Protein, His-tagged | Human | E.coli | His |
| ERBB4-3768H | Recombinant Human ERBB4 protein, GST-tagged | Human | E.coli | GST |
About EGF Receptors
The Epidermal Growth Factor (EGF) receptor, also known as ErbB1 or HER1, is a cell surface receptor that plays a critical role in cell growth, proliferation, and differentiation. It belongs to the ErbB family of receptor tyrosine kinases, which includes four members: EGFR (ErbB1, HER1), ErbB2 (HER2, neu in rodents), ErbB3 (HER3) and ErbB4 (HER4).
These structurally related receptors are single chain transmembrane glycoproteins consisting of an extracellular ligand-binding ectodomain, a transmembrane domain, a short juxtamembrane section, a tyrosine kinase domain and a tyrosine-containing C-terminal tail (Fig. 1A). The extracellular N-terminal domain contains four subdomains. The leucine-rich subdomains L1 and L2 directly interact with ligands. The cysteine-rich subdomain CR1 contains the dimerization loop responsible for receptor-receptor interaction. A short transmembrane and juxtamembrane domain links the extracellular domain to bilobed tyrosine kinase domain and the C-terminal tail. The binding of soluble ligands to the ectodomain of the receptor promotes homo- and heterodimer formation between receptors. Receptor dimerization is essential for activation of the intracellular tyrosine kinase domain and phosphorylation of the C-terminal tail. Phosphotyrosine residues then activate, either directly or through adaptor proteins, downstream components of signaling pathways including Ras/MAPK, PLCγ1/PKC, PI(3)kinase/Akt, and STAT pathways.
The EGF receptor is expressed in various tissues and cell types, including epithelial cells, fibroblasts, and immune cells. It is involved in the development and maintenance of epithelial tissues, wound healing, tissue repair, and the regulation of the immune response.
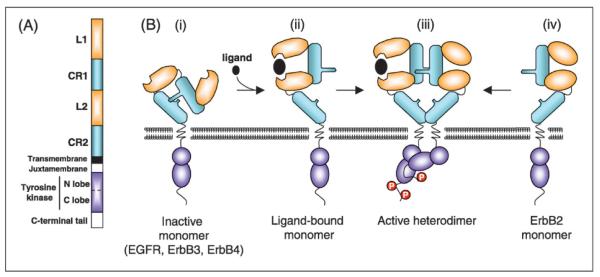 Fig.1 Structure of ErbB receptors. (Wieduwilt MJ, et al., 2008)
Fig.1 Structure of ErbB receptors. (Wieduwilt MJ, et al., 2008)
Signaling Pathways of the EGF Receptors
- Ras-Raf-MAPK Pathway: Upon ligand binding and receptor activation, the EGF receptor recruits and phosphorylates adaptor proteins, such as Grb2, which then bind to Son of Sevenless (SOS) protein. This interaction leads to the activation of Ras, a small GTPase. Activated Ras initiates a signaling cascade involving Raf kinases (Raf-1, A-Raf, B-Raf), MEK (MAPK/ERK kinase), and ERK (extracellular signal-regulated kinase). ERK translocates to the nucleus and phosphorylates various transcription factors, leading to gene expression changes that promote cell proliferation, survival, and differentiation.
- PI3K-Akt Pathway: The EGF receptor can activate phosphatidylinositol 3-kinase (PI3K) through direct binding or via adaptor proteins such as Grb2. Activated PI3K phosphorylates phosphatidylinositol-bisphosphate (PIP2) to generate phosphatidylinositol-triphosphate (PIP3). PIP3 recruits and activates Akt (also known as protein kinase B), which phosphorylates multiple downstream targets involved in cell survival, proliferation, and metabolism. Akt activation promotes cell growth and survival by inhibiting apoptosis and stimulating protein synthesis.
- STAT Pathway: Activation of the EGF receptor can also lead to the activation of signal transducers and activators of transcription (STAT) proteins. This occurs through the recruitment and activation of STAT proteins by the receptor or through the activation of other kinases downstream of the receptor. STAT proteins translocate to the nucleus, where they regulate the transcription of target genes involved in cell proliferation, differentiation, and immune responses.
These pathways are interconnected and can cross-talk with each other, leading to complex and integrated cellular responses. The activation of these pathways by the EGF receptor is tightly regulated and can be influenced by other signaling molecules and feedback mechanisms.
It's important to note that the signaling pathways activated by the EGF receptor can vary depending on cell type, context, and the presence of other co-receptors or signaling molecules. Additionally, aberrant activation or dysregulation of these pathways is associated with various diseases, including cancer, where they contribute to uncontrolled cell growth, survival, and metastasis.
Understanding the signaling pathways of the EGF receptor has provided valuable insights into cellular processes and has paved the way for the development of targeted therapies that aim to modulate these pathways in specific disease contexts.
Aberrant activation or overexpression of the EGF receptor has been implicated in the development and progression of various cancers. Mutations in the EGF receptor gene can lead to constitutive activation of the receptor, resulting in uncontrolled cell growth and tumor formation. Consequently, the EGF receptor has become an important target for cancer therapies. Drugs known as EGFR inhibitors, such as cetuximab and erlotinib, have been developed to block the activity of the receptor and inhibit tumor growth in certain types of cancer.
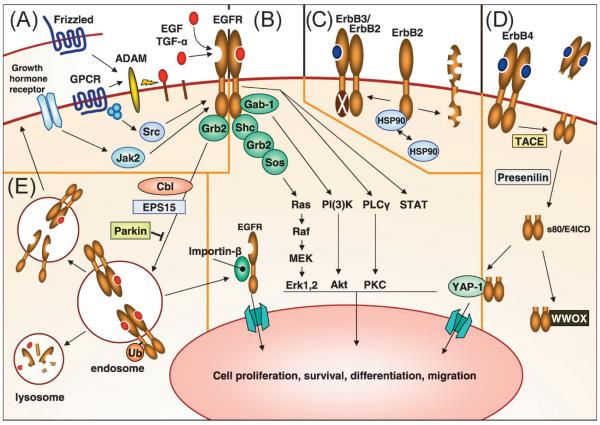 Fig.2 ErbB receptor signaling and regulation. (Wieduwilt MJ, et al., 2008)
Fig.2 ErbB receptor signaling and regulation. (Wieduwilt MJ, et al., 2008)
If you have any questions, requirements, or cooperation intentions, please feel free to contact us. We very much look forward to working with you and helping you achieve research and commercial success.
Related References
- Wieduwilt MJ, Moasser MM. The epidermal growth factor receptor family: biology driving targeted therapeutics. Cell Mol Life Sci. 2008;65(10):1566-1584.
- Murphrey MB, Quaim L, Rahimi N, Varacallo M. Biochemistry, Epidermal Growth Factor Receptor. In: StatPearls. Treasure Island (FL): StatPearls Publishing; December 3, 2023.
