SRF
-
Official Full Name
serum response factor (c-fos serum response element-binding transcription factor) -
Overview
This gene encodes a ubiquitous nuclear protein that stimulates both cell proliferation and differentiation. It is a member of the MADS (MCM1, Agamous, Deficiens, and SRF) box superfamily of transcription factors. This protein binds to the serum response element (SRE) in the promoter region of target genes. This protein regulates the activity of many immediate-early genes, for example c-fos, and thereby participates in cell cycle regulation, apoptosis, cell growth, and cell differentiation. This gene is the downstream target of many pathways; for example, the mitogen-activated protein kinase pathway (MAPK) that acts through the ternary complex factors (TCFs). -
Synonyms
SRF;serum response factor (c-fos serum response element-binding transcription factor);serum response factor;MCM1
Recombinant Proteins
- Human
- Mouse
- Chicken
- E.coli
- Insect Cells
- Mammalian Cells
- Wheat Germ
- HEK293
- Yeast
- His
- T7
- Non
- SUMO
- Avi
- Fc
- DDK
- Myc
- Flag
Background
What is SRF Protein?
SRF, or serum response factor, is a nuclear protein part of the big MADS box transcription factor family. It’s key for a bunch of life processes like cell growth, differentiation, cell death, and managing the cell cycle. By latching onto serum response elements (SRE) in gene promoters, SRF helps control the expression of immediate early genes (IEGs) and is crucial in mesoderm development, particularly in growing skeletal muscles. SRF is also tied to many signaling pathways and affects various cell functions. In cardiovascular diseases, SRF plays a big role in controlling genes linked to heart muscle. So, SRF is essential for things like embryonic development, tissue regeneration, muscle growth, and related disease progression.What is the Function of SRF Protein?
Serum Response Factor (SRF) is a key player in the MADS-box transcription factor family, showing up across various cell types like muscle, endothelial, fibroblast, liver, immune cells, and neurons. It’s crucial for tissue development in the heart, blood vessels, gut, and immune system, and it’s linked to diseases like cardiovascular issues and cancer. SRF docks onto serum response elements (SRE) in gene promoters to control gene transcription that drives important cell functions. It’s involved in helping cells grow, divide, and survive. Plus, it’s essential for developing embryos, muscles, and nervous system tissues, and it interacts with other transcription factors in pathways like Ras-ERK, SP1, ATF6, GATA4, and more, making it central to cell growth, differentiation, and other vital processes.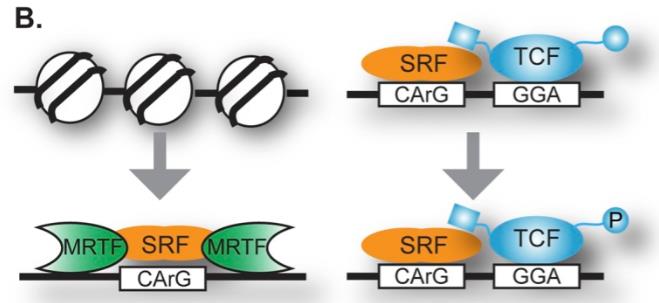
Fig1. In response to Rho-actin signaling, SRF binding to DNA is stimulated, which involves nucleosome displacement. (Kathleen A Clark, 2014)
SRF Related Signaling Pathway
SRF protein is key in pathways involving cell growth, differentiation, stress response, and building the cell’s structure. It teams up with transcription co-factors like TCF, MRTF, and p49/STRAP. For cell growth signals, SRF partners with TCF members, like Elk-1, which get activated by MAPK signals to push cell cycle gene expression. In stress situations, SRF kicks in via p38 MAPK to help transcribe genes like ANF in heart cells. MRTF-SRF interactions are critical for embryo development and cell shape changes, while SRF’s work with p49/STRAP influences mitochondria and cell structure. SRF also links with TEAD and YAP from the Hippo pathway, affecting heart fibrosis and muscle cell changes. Its activation is tweaked by miRNAs and lncRNAs, especially impacting the MRTF-SRF axis for structuring cells and gene control. Hence, SRF pathways are central to managing cell behavior, linking to heart development, disease, and cell-specific actions.SRF Related Diseases
Serum Response Factor, or SRF, is tightly linked to various diseases, notably heart diseases, cancer, and neurodegenerative conditions. It works by teaming up with various cofactors to regulate genes tied to contractile and actin cytoskeleton structures, crucial for heart and vascular health. Issues with SRF’s transcription activity can lead to congenital heart defects, like Tetralogy of Fallot. In heart diseases, the phosphorylation state of SRF is essential in conditions like pressure-induced hypertrophy and dilated cardiomyopathy. SRF also has a hand in cancer development and spread, though its influence can go both ways, potentially boosting or hindering tumor growth. In neurodegenerative diseases, excessive SRF might be linked to increased contractility in cerebellar arteries, possibly affecting Alzheimer’s. Additionally, SRF plays a role in gut health, and its absence could lead to developmental issues. Thus, SRF is a crucial player in these disorders, offering a significant target for research and treatment.Bioapplications of SRF
Recombinant SRF protein is a big deal in research, industry, and medicine. In research, it helps us explore crucial biological processes like cell cycles, apoptosis, and embryonic development, as well as regulate the expression of immediate early genes and muscle-specific genes. On the industrial side, cutting-edge biotech methods improve the production efficiency and quality of recombinant proteins, which in turn fuels new treatments and drug development. In medical research, SRF is linked with a variety of diseases, such as cardiovascular issues, cancer, and neurodegenerative disorders. It plays a role in smooth muscle cell phenotype switching and vascular remodeling, and its expression in gastric cancer provides key insights for both disease mechanics research and novel treatment approaches. So overall, recombinant SRF protein is super important for advancing both our scientific understanding and clinical applications in these areas.Case Study
Case Study 1: Ni H. et al. Arterioscler Thromb Vasc Biol. 2021
Vascular smooth muscle cell (VSMC) plasticity is key in atherosclerosis, and lncRNAs like CARMN play a role. CARMN, enriched in VSMCs, changes with atherosclerosis progression and is found in both mouse and human plaques. Reducing CARMN in mice led to a 38% decrease in atherosclerotic lesions and a 45% reduction in VSMC proliferation. CARMN influences VSMC behavior independently of miR143/145, interacting with SRF to alter gene expression. Knockdown of SRF eliminates CARMN’s effect on VSMC plasticity, highlighting its significance in this process.-
 Fig1. SRF and Histone H3 protein were detected by Western blotting.
Fig1. SRF and Histone H3 protein were detected by Western blotting. -
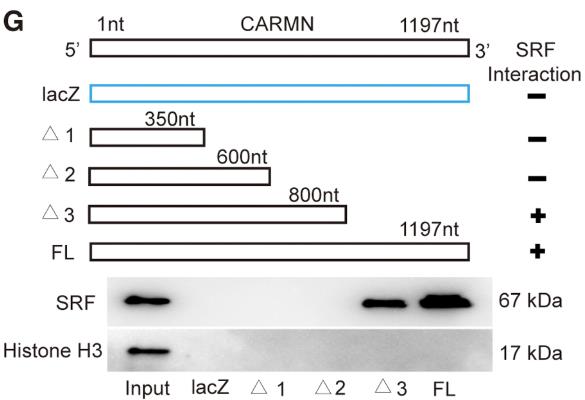 Fig2. Mapping the SRF interaction region of CARMN.
Fig2. Mapping the SRF interaction region of CARMN.
Case Study 2: Li J. et al. Circulation. 2020
Concentric and eccentric cardiac hypertrophy are linked to pressure and volume overload in cardiovascular disease, both raising heart failure risk. These hypertrophy forms show asymmetrical growth in cardiac myocytes, either in width or length. Understanding the mechanisms behind this could uncover new treatments. In studies involving rat myocytes, mice, and human tissue, a pathway was found that controls this uneven growth. It turns out that SRF phosphorylation acts as a switch, balancing myocyte width versus length. RSK3 and PP2A enzymes regulate SRF at signalosomes, influencing growth direction. Blocking these enzymes’ interactions with their scaffold alters cardiac remodeling, potentially guiding new heart failure therapies.-
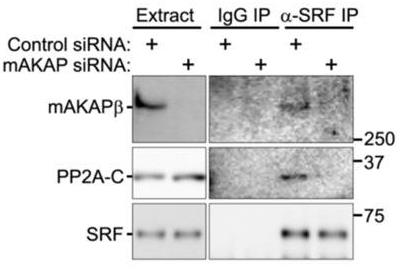 Fig3. Neonatal myocytes transfected with control or mAKAP siRNA were used for co-immunoprecipitation assay of endogenous SRF-mAKAPβ-PP2A complexes.
Fig3. Neonatal myocytes transfected with control or mAKAP siRNA were used for co-immunoprecipitation assay of endogenous SRF-mAKAPβ-PP2A complexes. -
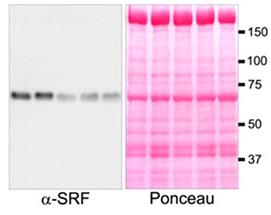 Fig4. Immunoblot signals for phospho-S103 and total SRF antibodies were normalized by total protein content detected by Ponceau S stain.
Fig4. Immunoblot signals for phospho-S103 and total SRF antibodies were normalized by total protein content detected by Ponceau S stain.
Quality Guarantee
High Purity
-
.jpg) Fig1. SDS-PAGE (SRF-2719H)
Fig1. SDS-PAGE (SRF-2719H)
-
.jpg) Fig2. SDS-PAGE (SRF-2460H)
Fig2. SDS-PAGE (SRF-2460H)
Involved Pathway
SRF involved in several pathways and played different roles in them. We selected most pathways SRF participated on our site, such as MAPK signaling pathway,cGMP-PKG signaling pathway,HTLV-I infection, which may be useful for your reference. Also, other proteins which involved in the same pathway with SRF were listed below. Creative BioMart supplied nearly all the proteins listed, you can search them on our site.
| Pathway Name | Pathway Related Protein |
|---|---|
| cGMP-PKG signaling pathway | CREB3L3,ATP2A3,SLC8A2,ATP1B3,IRS4,BDKRB2,GNA12,PLCB2,VDAC2,SLC25A4 |
| HTLV-I infection | CCND2,KAT2B,PRKACB,TNFRSF13C,MAP3K14,TNFRSF1A,PIK3CB,POLE2,GSK3B,TGFB1 |
| MAPK signaling pathway | PAK1,MAP3K7,PPP3CA,PRKCBB,PLA2G4F,CACNA1BA,CACNG2A,PPP3R2,FGF13A,MYCB |
| Viral carcinogenesis | HIST1H2BB,HIST1H4J,TBP,HLA-G,HIST1H4L,STAT5A,BAD,HIST1H2BL,SCRIB,HIST1H2BF |
-
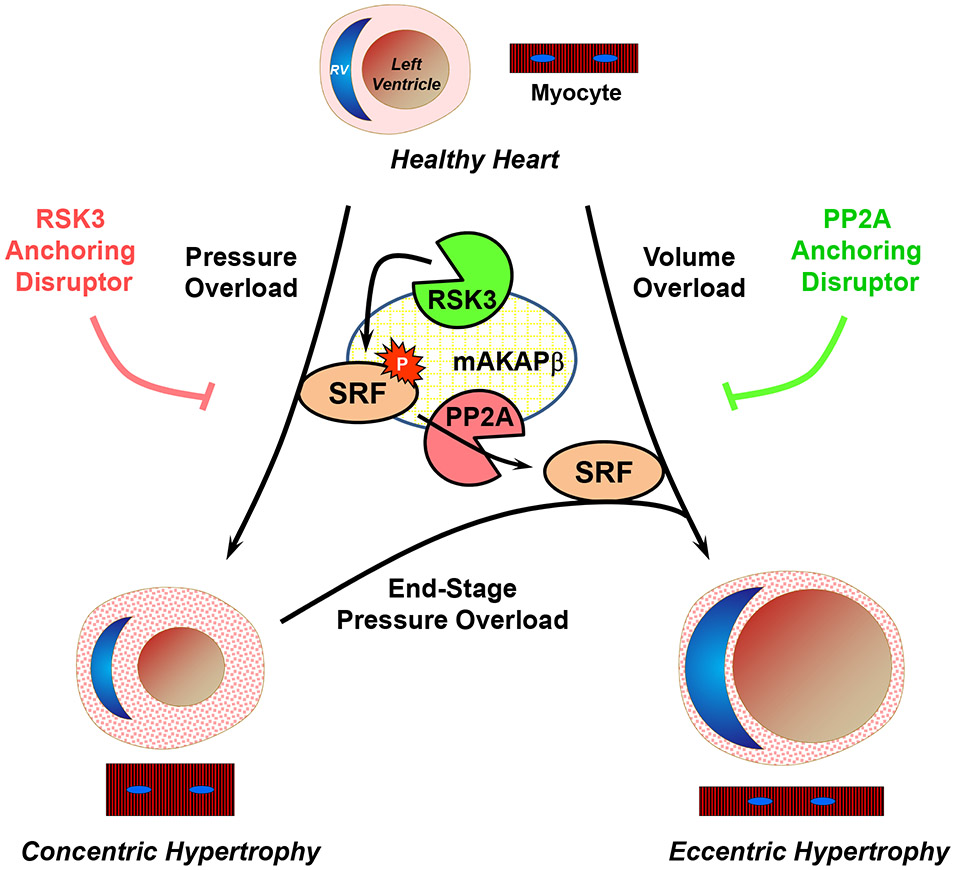 Fig1. Model for the role of SRF S103 phosphorylation in the regulation of pathological cardiac hypertrophy. (Jinliang Li, 2020)
Fig1. Model for the role of SRF S103 phosphorylation in the regulation of pathological cardiac hypertrophy. (Jinliang Li, 2020) -
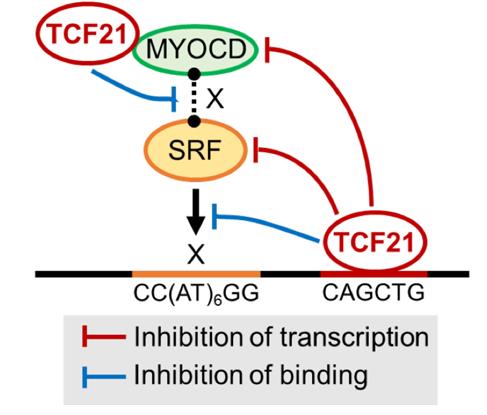 Fig2. TCF21 binding is enriched in SRF targeted loci genome-wide, and binds and inhibits transcription in both the SRF and MYOCD loci. (Manabu Nagao, 2020)
Fig2. TCF21 binding is enriched in SRF targeted loci genome-wide, and binds and inhibits transcription in both the SRF and MYOCD loci. (Manabu Nagao, 2020)
Protein Function
SRF has several biochemical functions, for example, RNA polymerase II core promoter proximal region sequence-specific DNA binding,chromatin DNA binding,histone deacetylase binding. Some of the functions are cooperated with other proteins, some of the functions could acted by SRF itself. We selected most functions SRF had, and list some proteins which have the same functions with SRF. You can find most of the proteins on our site.
| Function | Related Protein |
|---|---|
| transcription factor binding | RNF4,HES6,FLNA,TP53,MYBBP1A,FOS,DIP2A,JUNB,ARRB1,HMGA2 |
| histone deacetylase binding | NKX2-5,SFPQ,TCF21,CDC20,XBP1,RAD9A,CAMTA2,NACC2,RAD9,RFXANK |
| contributes_to transcription factor activity, RNA polymerase II core promoter sequence-specific | NKX3-1 |
| RNA polymerase II core promoter proximal region sequence-specific DNA binding | AGO1,RFX1A,NFYC,PHOX2B,FUBP3,FOS,NKX6-2,MYOG,NFIB,RFX5 |
| chromatin DNA binding | SLAMF7,INSM1A,GATA1,FOXC2,THRA,APEX1,NKAP,VAX2,VAX1,EZH2 |
| serum response element binding | NKX2-5,NKX2 |
| transcription factor activity, RNA polymerase II distal enhancer sequence-specific binding | THRA,ATF2,SOX10,PAX3,ATF1,LEF1,ZFHX3,SPI1,NFATC1,PKNOX1 |
| transcription factor activity, RNA polymerase II transcription factor binding | TWIST2,LHX2,GATA4,ZNF296,ATF4,SMAD4,ATF2,HIF1A,THRAA,MYOCD |
| protein binding | RAB7,CALCOCO1,SDCBP,PRNP,MLKL,DYX1C1,PEX19,AAK1,NLRC5,SAFB |
Interacting Protein
SRF has direct interactions with proteins and molecules. Those interactions were detected by several methods such as yeast two hybrid, co-IP, pull-down and so on. We selected proteins and molecules interacted with SRF here. Most of them are supplied by our site. Hope this information will be useful for your research of SRF.
MKL2;MKL1;MYOD1;MYOG;MYOCD;ALDH3A1;CIRBP;q8clf6_yerpe
Resources
Related Services
Related Products
References
- Olar, A; He, DD; et al. Biologic correlates and significance of axonogenesis in prostate cancer. HUMAN PATHOLOGY 45:1358-1364(2014).
- Shyu, KG; Cheng, WP; et al. Hypoxia activates muscle-restricted coiled-coil protein (MURC) expression via transforming growth factor-beta in cardiac myocytes. CLINICAL SCIENCE 126:367-375(2014).



