PISD
-
Official Full Name
phosphatidylserine decarboxylase -
Overview
Phosphatidylserine decarboxylases (PSDs; EC 4.1.1.65) catalyze the formation of phosphatidylethanolamine (PE) by;decarboxylation of phosphatidylserine (PS). Type I PSDs, such as PISD, are targeted to the inner mitochondrial;membrane by an N-terminal targeting sequence. PISD also contains a conserved LGST motif that functions as an;autocatalytic cleavage site where the proenzyme is split into mature alpha and beta subunits (Schuiki and Daum, 2009;(PubMed 19165886)). -
Synonyms
PISD;phosphatidylserine decarboxylase;phosphatidylserine decarboxylase proenzyme;dJ858B16.2;PSDC;PSD;PSSC;DJ858B16;DKFZp566G2246
Recombinant Proteins
- Human
- Zebrafish
- Mouse
- E.coli
- Wheat Germ
- Insect Cells
- Mammalian Cells
- In Vitro Cell Free System
- GST
- His
- SUMO
| Cat.# | Product name | Source (Host) | Species | Tag | Protein Length | Price |
|---|---|---|---|---|---|---|
| PISD-1731H | Recombinant Human PISD, GST-tagged | E.coli | Human | GST | 1-375aa | |
| PISD-15H | Recombinant Human PISD protein, GST-tagged | Wheat Germ | Human | GST | 1-409 a.a. |
|
| PISD-16H | Recombinant Human PISD protein, GST-tagged | Wheat Germ | Human | GST | 62-170 a.a. |
|
| PISD-17H | Recombinant Human PISD protein, His-tagged | Insect Cells | Human | His | 1-375 a.a. |
|
| PISD-4892Z | Recombinant Zebrafish PISD | Mammalian Cells | Zebrafish | His |
|
|
| PISD-001H | Recombinant Human PISD Protein, His-tagged | E.coli | Human | His | Full L. |
|
| PISD-18H | Recombinant Human PISD Protein, His/SUMO-tagged | E.coli | Human | His&SUMO | 1-375aa |
|
| Pisd-1940M | Recombinant Mouse Pisd Protein, His-tagged | E.coli | Mouse | His | Met1-Leu406 |
|
| PISD-625HF | Recombinant Full Length Human PISD Protein, GST-tagged | In Vitro Cell Free System | Human | GST | Full L. 409 amino acids |
|
Background
What is PISD Protein?
PISD protein is an enzyme found in mitochondria, playing a key role in keeping our cells in check. It’s responsible for transforming phosphatidylserine into phosphatidylethanolamine (PE), which is crucial for good cell membrane health and making sure mitochondria do their job right. When there’s a glitch with PISD protein, it can mess up how energy is produced in cells, leading to problems associated with mitochondrial diseases. This disruption can affect overall cell health, causing various health issues down the road.What is the Function of PISD Protein?
The PISD protein is like a behind-the-scenes worker in our cells, mainly hanging out in the mitochondria—the cell's powerhouse. Its main job is converting a molecule called phosphatidylserine into phosphatidylethanolamine (PE). PE is super important for maintaining healthy cell membranes and making sure the mitochondria can crank out energy efficiently. When the PISD protein isn’t functioning right, it can throw off the cell’s energy production, potentially leading to mitochondrial-related health issues and affecting overall cell health.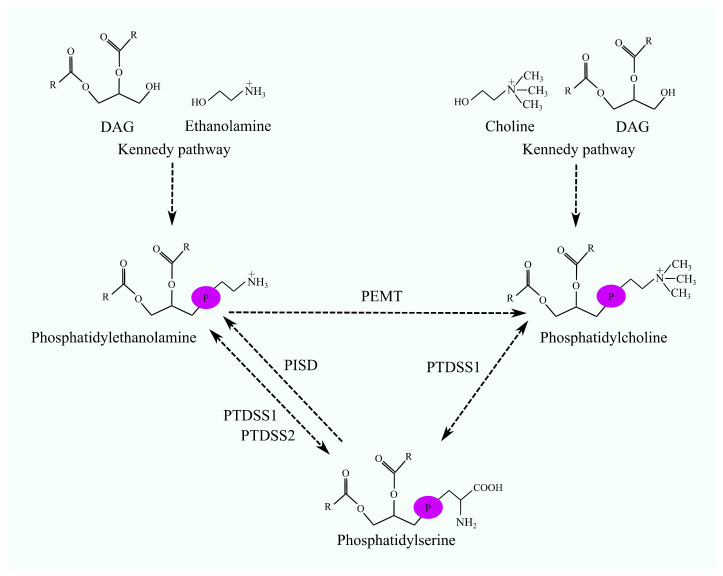
Fig1. The interconversions among PC, PE, and PS. PC and PE are synthesized via the Kennedy pathway. (Jan Korbecki, 2024)
PISD Related Signaling Pathway
The PISD-related signaling pathway is all about how this protein influences critical processes within the cell, especially in the mitochondria. PISD is crucial for converting phosphatidylserine into phosphatidylethanolamine (PE), and this conversion is vital for maintaining cell membrane integrity and mitochondrial function. When PISD is doing its job right, everything runs smoothly, with balanced energy production and proper cellular communication. But if there’s a hiccup in this pathway, it can disrupt how signals are sent across cells, leading to problems with mitochondrial efficiency and overall cellular health. This disruption can sometimes snowball into larger health issues, particularly those involving energy deficits.PISD Related Diseases
When PISD doesn't work properly, it can lead to a bunch of health issues because mitochondria aren’t able to produce energy efficiently. This enzyme is vital for keeping cell membranes stable by converting certain molecules, and without it running smoothly, several diseases might pop up. For instance, problems with PISD can cause mitochondrial diseases, which affect energy levels and muscle function, and might also contribute to nerve-related disorders. It's pretty much about cells struggling to keep up with energy demands, leading to various symptoms depending on which body's systems are hit hardest.Bioapplications of PISD
PISD has some interesting bioapplications because of its role in maintaining healthy cells and energy production. Since this protein is crucial for turning phosphatidylserine into phosphatidylethanolamine, scientists are looking into ways to harness its function for therapeutic uses. For example, boosting PISD activity could potentially help treat mitochondrial disorders and improve cellular energy efficiency, offering hope for diseases linked to energy deficits. Additionally, understanding how PISD works might lead to innovations in cell biology and new treatments that ensure cells maintain their structure and function properly. It’s all about leveraging what PISD does best to enhance health outcomes.Case Study
Case Study 1: Kumar S. et al. Biol Open. 2021
Phosphatidylethanolamine is a key player in cell membranes, influencing organelle dynamics. One way it’s made is by decarboxylating phosphatidylserine using an enzyme found in mitochondria. Researchers studied another form, PISD-LD, which comes from a splice variant and goes to lipid droplets and mitochondria. Its location depends on a specific segment influenced by diet. When conditions favor lipid storage, PISD-LD targets droplets, but during lipid use, it shifts to mitochondria. Lacking both enzyme forms hampers fat storage, highlighting their essential role in managing lipids.-
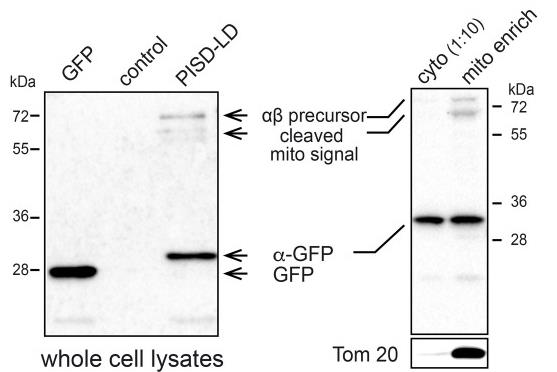 Fig1. PISD-LD-GFP is proteolytically processed in HeLa cells and a small proportion of it co-purifies with mitochondria.
Fig1. PISD-LD-GFP is proteolytically processed in HeLa cells and a small proportion of it co-purifies with mitochondria. -
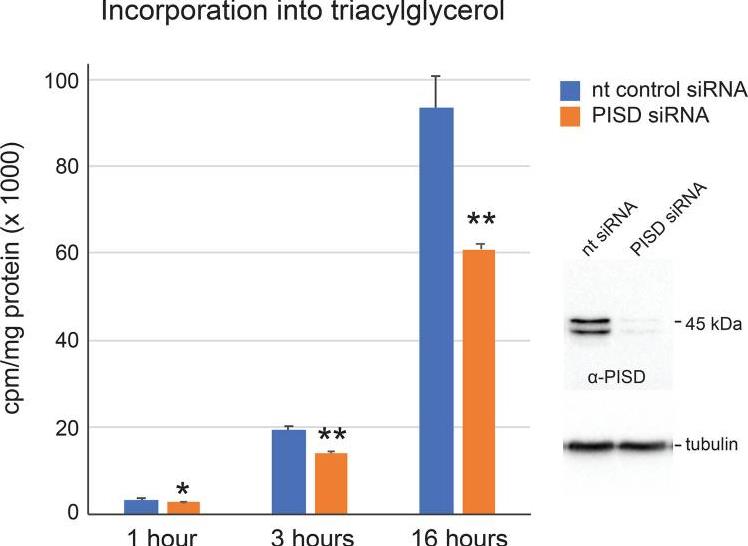 Fig2. Decreased triacylglycerol synthesis by PISD RNAi cells.
Fig2. Decreased triacylglycerol synthesis by PISD RNAi cells.
Case Study 2: Zhao T. et al. Life Sci Alliance. 2019
Two sisters with cataracts and short stature showed PISD gene mutations affecting an enzyme in mitochondria. Their cells had fragmented mitochondria and larger lysosomes. Treatments helped restore normal structures. The gene variants caused different disruptions to the enzyme, linking their symptoms to mitochondrial disorders.-
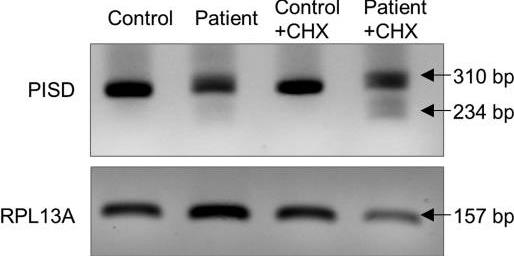 Fig3. Evidence for alternative splicing and nonsense mediated decay (NMD) of PISD mRNA in patient fibroblasts.
Fig3. Evidence for alternative splicing and nonsense mediated decay (NMD) of PISD mRNA in patient fibroblasts. -
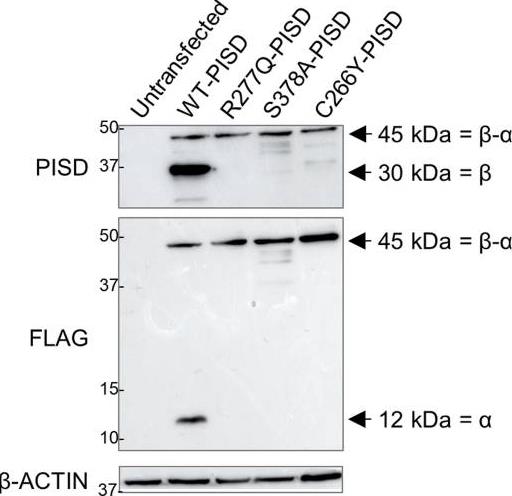 Fig4. Impaired autocatalytic proteolysis of R277Q and C266Y pathogenic PISD variants.
Fig4. Impaired autocatalytic proteolysis of R277Q and C266Y pathogenic PISD variants.
Involved Pathway
PISD involved in several pathways and played different roles in them. We selected most pathways PISD participated on our site, such as Glycerophospholipid metabolism,Metabolic pathways, which may be useful for your reference. Also, other proteins which involved in the same pathway with PISD were listed below. Creative BioMart supplied nearly all the proteins listed, you can search them on our site.
| Pathway Name | Pathway Related Protein |
|---|---|
| Metabolic pathways | TYR,COX15,CKMT1A,PNAT3,GCLC,SMPD2,ALPP,AKR1A1B,ETNK2,IDH2 |
| Glycerophospholipid metabolism | LYCAT,CHKA,AGPAT1,MBOAT2,CHAT,GPD1L,CDS2,PCYT1AB,GPD1,MBOAT1 |
Protein Function
PISD has several biochemical functions, for example, phosphatidylserine decarboxylase activity. Some of the functions are cooperated with other proteins, some of the functions could acted by PISD itself. We selected most functions PISD had, and list some proteins which have the same functions with PISD. You can find most of the proteins on our site.
| Function | Related Protein |
|---|---|
| phosphatidylserine decarboxylase activity | GOT1 |
Interacting Protein
PISD has direct interactions with proteins and molecules. Those interactions were detected by several methods such as yeast two hybrid, co-IP, pull-down and so on. We selected proteins and molecules interacted with PISD here. Most of them are supplied by our site. Hope this information will be useful for your research of PISD.
DHX29;DAGLB
Resources
Related Services
Related Products
References
- Pierucci, D; Cicconi, S; et al. NGF-withdrawal induces apoptosis in pancreatic beta cells in vitro. DIABETOLOGIA 44:1281-1295(2001).
- Dowhan, W; et al. A retrospective: Use of Escherichia coli as a vehicle to study phospholipid synthesis and function. BIOCHIMICA ET BIOPHYSICA ACTA-MOLECULAR AND CELL BIOLOGY OF LIPIDS 1831:471-494(2013).


