MAX
-
Official Full Name
MYC associated factor X -
Overview
The protein encoded by this gene is a member of the basic helix-loop-helix leucine zipper (bHLHZ) family of transcription factors. It is able to form homodimers and heterodimers with other family members, which include Mad, Mxi1 and Myc. Myc is an oncoprotein implicated in cell proliferation, differentiation and apoptosis. The homodimers and heterodimers compete for a common DNA target site (the E box) and rearrangement among these dimer forms provides a complex system of transcriptional regulation. Mutations of this gene have been reported to be associated with hereditary pheochromocytoma. A pseudogene of this gene is located on the long arm of chromosome 7. Alternative splicing results in multiple transcript variants. [provided by RefSeq, Aug 2012] -
Synonyms
MAX;MYC associated factor X;bHLHd4;protein max;class D basic helix-loop-helix protein 4
Recombinant Proteins
- Human
- Rhesus macaque
- Rat
- Zebrafish
- Mouse
- E.coli
- HEK293
- Wheat Germ
- Mammalian Cells
- Insect Cells
- Insect cells
- Flag
- His
- DDK
- Myc
- GST
- Non
- Avi
- Fc
Background
What is MAX Protein?
MAX protein, or Myc-Associated Factor X, is a transcription factor from the bHLHZ family. It can link up with MYC or MAD proteins to form complexes that bind to a specific DNA sequence, 5'-CAC[GA]TG-3'. When MAX joins MYC, they activate transcription, but with MAD, they repress it. MAX is found in high amounts in the brain, heart, and lungs, while it's less present in the liver, kidneys, and muscles. It’s crucial for processes like cell growth, differentiation, and apoptosis, and is involved in developing various cancers.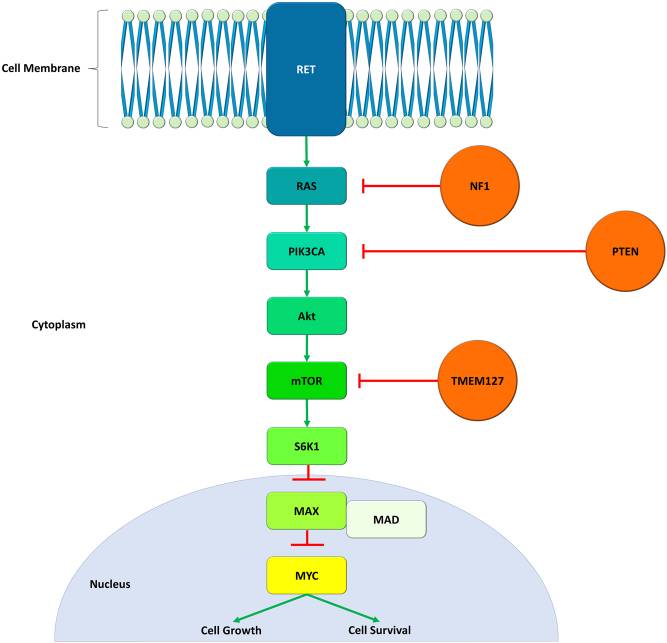
Fig1. The role of MAX in tumorigenesis. (Paul Benjamin Loughrey, 2022)
What is the Function of MAX Protein?
MAX protein, or Myc-Associated Factor X, is an influential player in gene activity control. Belonging to the basic helix-loop-helix leucine zipper (bHLHLZ) family, this protein tags along with MYC protein to create something called a heterodimer. This duo binds to a specific DNA spot called the E-box sequence (5’-CAC[GA]TG-3’), sparking the start of gene transcription. But that’s not all—MAX can also team up with MAD protein to suppress gene transcription. This protein is crucial in the processes of cell growth, differentiation, and even programmed cell death. On top of that, MAX takes part in chromatin remodeling by rallying complexes with H3 “Lys-9” histone methyltransferase activity to keep MYC’s transcriptional activity in check. Essentially, MAX is like a conductor in the orchestra of cellular functions, making sure everything is in harmony.MAX Related Signaling Pathway
The MAX protein is quite active across various signaling pathways, mainly by teaming up with proteins from the MYC family to regulate how genes are expressed. When it pairs with c-Myc, it helps kickstart the transcription of E-box sequences, which in turn promotes cell growth and differentiation. But MAX doesn’t stop there; it can also form inhibitory complexes with members of the MAD protein family, putting the brakes on gene transcription mediated by MYC. This regulatory setup makes MAX a pivotal player in processes like cell growth, differentiation, and apoptosis, and it’s crucial in the onset and progression of cancer. It’s like MAX is wearing multiple hats, balancing cell life activities, and making sure things don’t go haywire.MAX Related Diseases
MAX protein is linked to various diseases, playing a particularly significant role in cancer. When MAX combines with c-Myc to form heterodimers, it regulates gene expression and influences how cells grow and die, making it pivotal in the onset and progression of many types of cancer. Beyond cancer, MAX is also tied to osteoarthritis; its interacting partner, MXI1, induces an imbalance of IL-17-producing T-helper/regulatory T-cells in osteoarthritis by upregulating TCTN2. These findings indicate that MAX and its interacting proteins are biologically significant in the mechanisms of disease development and could serve as potential therapeutic targets. Understanding and targeting these pathways might offer new avenues for treating these complex diseases, adding another layer to how we might approach both cancer and autoimmune conditions like osteoarthritis.Bioapplications of MAX
The MAX protein finds a multitude of applications in scientific research, industrial production, and clinical studies. In the research world, MAX is widely used as the key partner of MYC to explore its role in cell proliferation, differentiation, and apoptosis. Scientists also delve into MAX’s function in cancer, such as regulating tumor cell growth through its interaction with MYC. On the industrial front, recombinant forms of MAX protein can be expressed using hosts like yeast, aiding in the production of lab reagents and research tools. Clinically, the expression levels of MAX in certain cancers serve as potential biomarkers, helping to gauge disease progression and treatment response. This versatility makes MAX an invaluable tool across different fields, providing insights into biological processes and offering potential pathways for monitoring and treating diseases.Case Study
Case Study 1: Wang D. et al. Nucleic Acids Res. 2017
The cancer-related transcription factor MYC teams up with MAX to regulate genes by attaching to DNA at spots called E-boxes (5΄-CACGTG-3΄). In mammals, the central CpG of the E-box can be modified in several ways, like methylation, but MAX prefers binding to unmodified or 5caC-modified versions. When crystallized with a 5caC E-box, it's shown that MAX uses certain arginines (Arg36 and Arg60) to recognize 5caC. In over 800 cases of multiple myeloma, about 3% showed changes in MAX, often at key spots important for DNA binding, particularly affecting Arg35, Arg36, and Arg60. Lab studies revealed mutations in Arg36 or Arg35 stopped DNA binding, while changes in Arg60 reduced it but kept a preference for the 5caC E-box. Interestingly, these MAX mutations identify myeloma patients with lower MYC levels and better overall outcomes.-
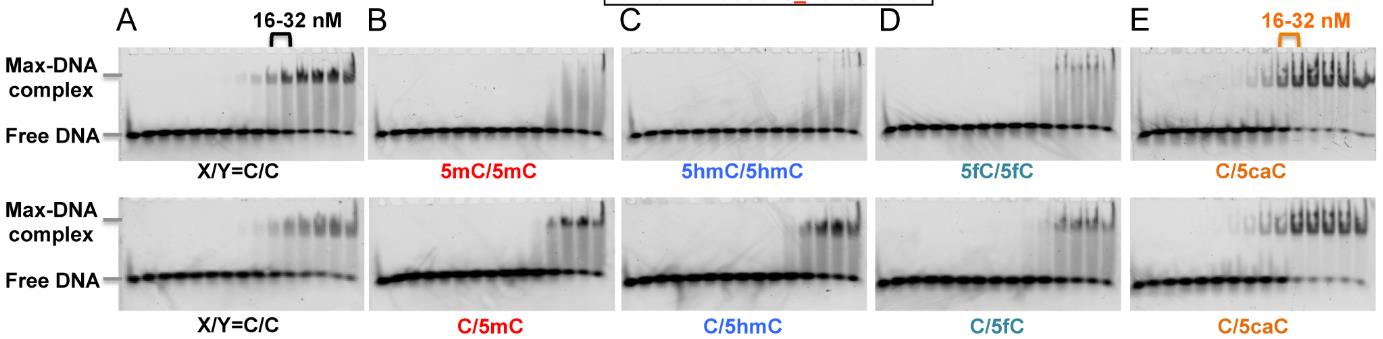 Fig1. Electrophoretic mobility shift assay of full length MAX protein binding to oligonucleotides containing a single E-box.
Fig1. Electrophoretic mobility shift assay of full length MAX protein binding to oligonucleotides containing a single E-box. -
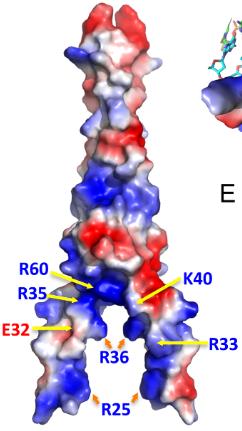 Fig2. The surface charge of MAX homodimer at neutral pH is displayed as blue for positive, red for negative, and white for neutral.
Fig2. The surface charge of MAX homodimer at neutral pH is displayed as blue for positive, red for negative, and white for neutral.
Case Study 2: Xu J. et al. J Am Chem Soc. 2009
Max-E47 is a custom-made hybrid protein combining parts of Max and E47 proteins. It's designed to latch onto the E-box site (5'-CACGTG) and strongly activate transcription in yeast systems, a function usually handled by Myc/Max/Mad networks. Two versions, Max-E47Y and Max-E47YF, activate the E-box but not as strongly. Tests showed these proteins bind tightly to the E-box, with very low nanomolar affinity. Interestingly, these hybrids don't have an activation domain and don't interact with c-Myc, meaning they could act as blockers to prevent activation of certain E-box-responsive genes, especially those targeted by the c-Myc/Max complex. Experiments demonstrated that Max-E47 can outcompete natural Max proteins for the E-box, while the mutated versions are less competitive. This setup offers a basic framework to further study how protein structures relate to DNA-binding and might be useful in therapies or as biochemical tools targeting the E-box.-
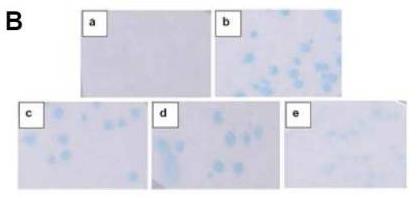 Fig3. The X-gal colony-lift filter assays of Max-E47 hybrids.
Fig3. The X-gal colony-lift filter assays of Max-E47 hybrids. -
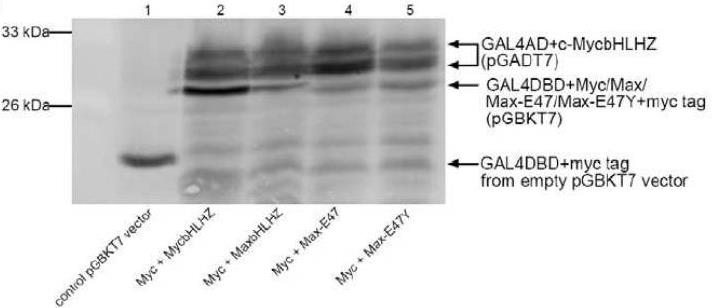 Fig4. Western blot of Y2H.
Fig4. Western blot of Y2H.
Quality Guarantee
High Purity
-
.jpg) Fig1. SDS-PAGE (MAX-1547H)
Fig1. SDS-PAGE (MAX-1547H) -
.jpg) Fig2. SDS-PAGE (MAX-3233H)
Fig2. SDS-PAGE (MAX-3233H)
Involved Pathway
MAX involved in several pathways and played different roles in them. We selected most pathways MAX participated on our site, such as MAPK signaling pathway,Pathways in cancer,Transcriptional misregulation in cancer, which may be useful for your reference. Also, other proteins which involved in the same pathway with MAX were listed below. Creative BioMart supplied nearly all the proteins listed, you can search them on our site.
| Pathway Name | Pathway Related Protein |
|---|---|
| MAPK signaling pathway | RPS6KAL,PAK1,CACNG2A,PTPRR,MAP3K5,RAF1,MAP3K6,MAPK6,FGF4,TGFB1A |
| Pathways in cancer | GNG3,MMP1A,ITGAV,MTOR,FZD10,TCF7,WNT1,WNT5B,TPR,PIK3CB |
| Small cell lung cancer | SKP2,TP53,RARB,NFKBIA,TRP53,TRAF2,LAMB1,BCL2L1,TRAF5,PTK2 |
| Transcriptional misregulation in cancer | MAF,HIST1H3G,PTCRA,ERG,NTRK1,PLAU,ZEB1,NFKB1,SP1,BCL2L1 |
-
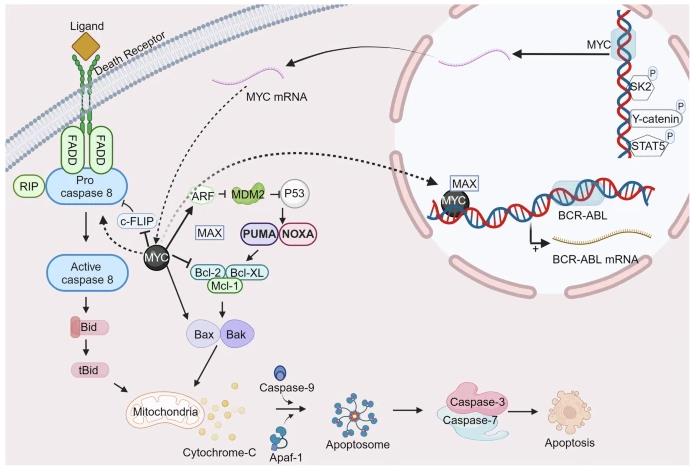 Fig1. Schematic representation of the c-Myc/Max signaling pathway. (Hossam Nada, 2024)
Fig1. Schematic representation of the c-Myc/Max signaling pathway. (Hossam Nada, 2024) -
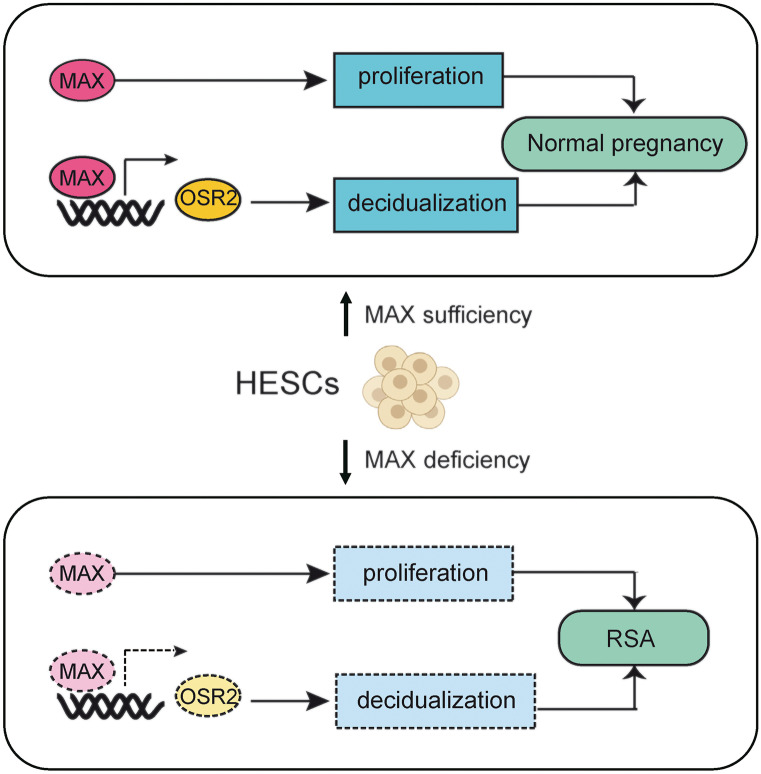 Fig2. Schematic illustration of the mechanism of the roles of MAX in endometrium of women. (Weixu Ma, 2022)
Fig2. Schematic illustration of the mechanism of the roles of MAX in endometrium of women. (Weixu Ma, 2022)
Protein Function
MAX has several biochemical functions, for example, RNA polymerase II core promoter proximal region sequence-specific DNA binding,protein binding,protein dimerization activity. Some of the functions are cooperated with other proteins, some of the functions could acted by MAX itself. We selected most functions MAX had, and list some proteins which have the same functions with MAX. You can find most of the proteins on our site.
| Function | Related Protein |
|---|---|
| transcription factor activity, sequence-specific DNA binding | TCF12,HOXC4A,SOX32,SOX6,IKZF3,TRIM22,PRRXL1,ZNF480,GATAD1,RUNX2A |
| RNA polymerase II core promoter proximal region sequence-specific DNA binding | NKX6-2,DDN,LEF1,SRF,NKX2,MAFB,FOSB,MYF5,FOXA2,SMAD4 |
| transcription coactivator activity | MYCBP,JMY,MED1,ESRRB,PSMD9,DDX5,TUBB3,CCNE1,RXRB,YAF2 |
| transcription factor activity, RNA polymerase II core promoter sequence-specific | SMAD3,HEY2,TFAP2B,HEYL,MEF2C,PAX7,HEY1,NKX3 |
| protein dimerization activity | STK4,SMG1,PIP4K2AA,ATOH1A,PTF1A,POLR2J2,MYC,MYCLB,RIMKLB,TXLNB |
| protein binding | NPHS1,ZNF524,TBL3,ITGB3BP,SRI,DDOST,KAT7,LYL1,GPM6A,HADHA |
| transcription cofactor activity | CREB1,PHF21A,MSX2,ANKRA2,TADA2A,LDB2B,NFKBIZ,ELK4,KAT2B,ZFP667 |
Interacting Protein
MAX has direct interactions with proteins and molecules. Those interactions were detected by several methods such as yeast two hybrid, co-IP, pull-down and so on. We selected proteins and molecules interacted with MAX here. Most of them are supplied by our site. Hope this information will be useful for your research of MAX.
MYC
Resources
Research Area
Related Services
Related Products
References
- Soleri, R; Demey, H; et al. Peptide conjugated chitosan foam as a novel approach for capture-purification and rapid detection of hapten - Example of ochratoxin A. BIOSENSORS & BIOELECTRONICS 67:634-641(2015).
- Henriksen, LT; Harila-Saari, A; et al. PEG-Asparaginase Allergy in Children With Acute Lymphoblastic Leukemia in the NOPHO ALL2008 Protocol. PEDIATRIC BLOOD & CANCER 62:427-433(2015).



