GSR
-
Official Full Name
glutathione reductase -
Overview
This gene encodes a member of the class-I pyridine nucleotide-disulfide oxidoreductase family. This enzyme is a homodimeric flavoprotein. It is a central enzyme of cellular antioxidant defense, and reduces oxidized glutathione disulfide (GSSG) to the sulfhydryl form GSH, which is an important cellular antioxidant. Rare mutations in this gene result in hereditary glutathione reductase deficiency. Multiple alternatively spliced transcript variants encoding different isoforms have been found. [provided by RefSeq, Aug 2010] -
Synonyms
GSR;glutathione reductase;HEL-75;glutathione reductase, mitochondrial;GR;GRase;epididymis luminal protein 75
Recombinant Proteins
- Human
- Zebrafish
- Mouse
- Rat
- E.coli
- Mammalian Cells
- Wheat Germ
- HEK293
- In Vitro Cell Free System
- Non
- His
- GST
- T7
- DDK
- Myc
- Avi
- Fc
Background
What is GSR protein?
GSR (glutathione-disulfide reductase) gene is a protein coding gene which situated on the short arm of chromosome 8 at locus 8p12. GSR is an enzyme encoded by the GSR gene in humans. It plays a central role in cellular antioxidant defense by reducing oxidized glutathione disulfide (GSSG) to its reduced form, glutathione (GSH). This process is crucial for maintaining the intracellular balance of GSH, which is essential for various cellular functions, including protection against oxidative stress and detoxification of harmful substances. The protein's subcellular localization can vary, and it has been found in different cellular compartments such as the cytoplasm, mitochondria, and nucleus, reflecting its diverse functions within the cell. GSR protein is consisted of 522 amino acids and its molecular mass is approximately 56.3 kDa.
What is the function of GSR protein?
GSR protein is involved in the reduction of oxidized glutathione disulfide (GSSG) to its reduced form, glutathione (GSH). Glutathione is the most abundant non-protein thiol in cells and is a crucial antioxidant that helps protect cells from oxidative damage caused by reactive oxygen species (ROS). GSR plays a role in the detoxification process by helping to regenerate GSH, which is used in the conjugation and removal of toxic compounds, including drugs and environmental toxins. The protein contributes to the proper functioning of the immune system, as a balanced redox state is necessary for the immune cells to perform their functions effectively. GSR is involved in various metabolic pathways, including the metabolism of drugs and xenobiotics, where it helps in the reduction of certain compounds to facilitate their metabolism and excretion.
GSR Related Signaling Pathway
The reduced glutathione maintained by GSR can modulate the activity of various signaling molecules and enzymes, thereby influencing cellular signaling pathways. In the context of G-protein-coupled receptors (GPCRs), GSR may be indirectly involved in signal transduction pathways that are activated by GPCRs. GPCRs can activate a variety of downstream signals depending on their signaling partners, and GSR could potentially be part of these pathways, particularly those related to oxidative stress and immune response. GSR protein, by reducing GSSG to GSH, contributes to the cell's defense against oxidative stress. This pathway is crucial for neutralizing reactive oxygen species (ROS) that can damage cellular components. The reduced glutathione (GSH) maintained by GSR is involved in the detoxification of harmful substances, including drugs and environmental toxins, by facilitating their conjugation with glutathione.
GSR Related Diseases
The GSR protein, Glutathione Reductase, has been implicated in a variety of diseases, including oxidative stress, cellular antioxidant defense, and metabolic regulation. GSR protein-related diseases include liver diseases (such as acute hepatitis, drug-induced liver injury, cirrhosis, and liver cancer), neonatal jaundice, certain types of cancer (including oxidative stress status of tumors, drug resistance, growth, and metastasis), and hereditary glutathione reductase deficiency. In addition, GSR protein is also associated with type 2 diabetes, IgA nephropathy, arthritis, multiple myeloma, autosomal dominant polycystic kidney disease, glioblastoma multiforme, focal segmental glomerulosclerosis, Parkinson's disease, chronic kidney disease, bronchitis, astrocytoma, lymphoma, ulcerative colitis, inflammatory bowel disease, rheumatoid arthritis, diabetic neuropathy, diabetic kidney Disease, systemic lupus erythematosus, obesity, gastrointestinal diseases, autoimmune diseases, hereditary spherocytosis, non-Hodgkin lymphoma, melanoma and other diseases. In these diseases, GSR proteins may play a role by influencing REDOX balance, cell proliferation, apoptosis, and drug sensitivity.

Fig1. Proposed mechanistic model of sorafenib resistance caused by SLC27A5 deficiency. (Feng-Li Xu, 2023)
Bioapplications of GSR
In drug target research, GSR can serve as an important drug target to help scientists discover and develop new drugs, especially in the field of antioxidant and anti-inflammatory drugs. Among therapeutic strategies, GSR may help boost the antioxidant capacity of cells to fight diseases caused by oxidative stress. In the study of plant stress resistance, GSR may be used to improve the antioxidant capacity of crops in response to environmental stress. During cell culture, GSR may be used to maintain a healthy state of cells, especially in cases where antioxidant protection is required.
Case Study
Case Study 1: Soma Jobbagy, 2019
Cells evolved robust homeostatic mechanisms to protect against oxidation or alkylation by electrophilic species. Glutathione (GSH) is the most abundant intracellular thiol, protects cellular components from oxidation and is maintained in a reduced state by glutathione reductase (GR). Nitro oleic acid (NO2-OA) is an electrophilic fatty acid formed under digestive and inflammatory conditions that both reacts with GSH and induces its synthesis upon activation of Nrf2 signaling. The effects of NO2-OA on intracellular GSH homeostasis were evaluated. In addition to upregulation of GSH biosynthesis, the researchers observed that NO2-OA increased intracellular GSSG in an oxidative stress-independent manner. Akin to NO2-OA, the electrophilic Nrf2 activators bardoxolone-imidazole (CDDO-Im), bardoxolone-methyl (CDDO-Me) and dimethyl fumarate (DMF) also upregulated GSH biosynthesis while promoting GSSG accumulation, but without directly inhibiting GR activity. In vitro assays in which GR was treated with increasing GSH concentrations and GSH depletion experiments in cells revealed that GR activity is finely regulated via product inhibition, an observation further supported by theoretical (kinetic modeling of cellular GSSG:GSH levels) approaches.

Fig1. NO2-OA is a reversible covalent inhibitor of GR.
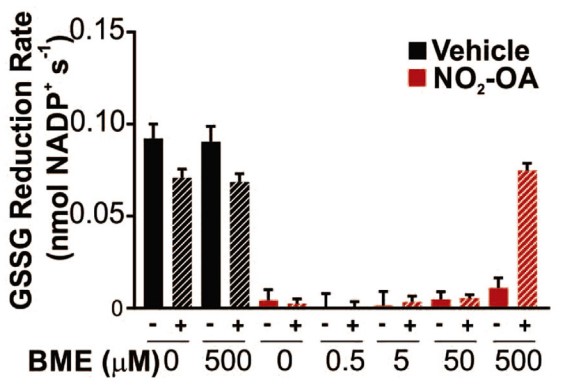
Fig2. GR activity restoration is dependent of BME concentration.
Case Study 2: José Antonio Mora-Lorca, 2016
Glutathione is the most abundant thiol in the vast majority of organisms and is maintained in its reduced form by the flavoenzyme glutathione reductase. In this work, the researchers describe the genetic and functional analysis of the Caenorhabditis elegans gsr-1 gene that encodes the only glutathione reductase protein in this model organism. By using green fluorescent protein reporters they demonstrate that gsr-1 produces two GSR-1 isoforms, one located in the cytoplasm and one in the mitochondria. gsr-1 loss of function mutants display a fully penetrant embryonic lethal phenotype characterized by a progressive and robust cell division delay accompanied by an aberrant distribution of interphasic chromatin in the periphery of the cell nucleus. Maternally expressed GSR-1 is sufficient to support embryonic development but these animals are short-lived, sensitized to chemical stress, have increased mitochondrial fragmentation and lower mitochondrial DNA content. Furthermore, the embryonic lethality of gsr-1 worms is prevented by restoring GSR-1 activity in the cytoplasm but not in mitochondria.
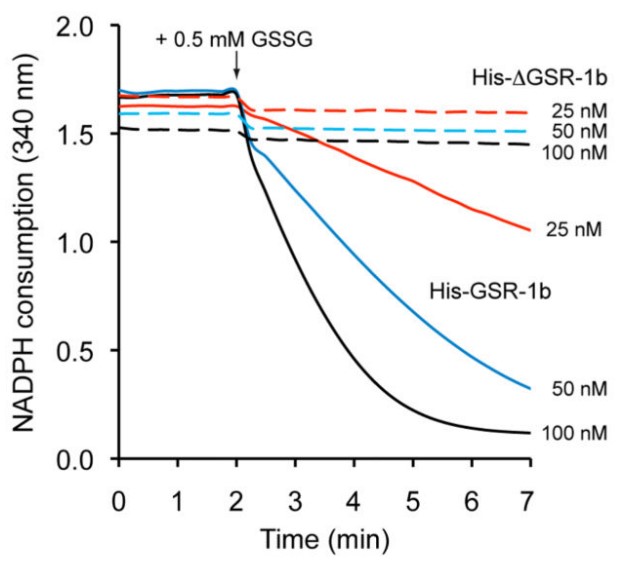
Fig3. GSSG reductase enzymatic activity of different concentrations of recombinant His-GSR-1b (straight lines) and His-ΔGSR-1b (dashed lines).
 embryonic lethal phenotype is rescued when GSR-1 activity is restored in the cytoplasm but not in mitochondria.jpg)
Fig4. The gsr-1(tm3574) embryonic lethal phenotype is rescued when GSR-1 activity is restored in the cytoplasm but not in mitochondria.
Quality Guarantee
High Purity
.jpg)
Fig1. SDS-PAGE (GSR-4404H)
.
.jpg)
Fig2. SDS-PAGE (GSR-3139H)
Involved Pathway
GSR involved in several pathways and played different roles in them. We selected most pathways GSR participated on our site, such as Glutathione metabolism,Thyroid hormone synthesis, which may be useful for your reference. Also, other proteins which involved in the same pathway with GSR were listed below. Creative BioMart supplied nearly all the proteins listed, you can search them on our site.
| Pathway Name | Pathway Related Protein |
|---|---|
| Glutathione metabolism | GPX4B,GSTM,GPX4A,GSTP2,GSTT1,GGT6,GGCTB,GSTA1,GSTK1,PGD |
| Thyroid hormone synthesis | CREB5,SLC26A4,DUOXA2,GNAQ,TG,CREB3L2,GPX8,PLCB1,ATP1B4,TPO |
Protein Function
GSR has several biochemical functions, for example, NADP binding,electron carrier activity,flavin adenine dinucleotide binding. Some of the functions are cooperated with other proteins, some of the functions could acted by GSR itself. We selected most functions GSR had, and list some proteins which have the same functions with GSR. You can find most of the proteins on our site.
| Function | Related Protein |
|---|---|
| NADP binding | DPYD,NOS1,MTHFR,ME1,NOS3,NOX5,NNT,FMO1,MTRR,G6PDX |
| flavin adenine dinucleotide binding | ACADSB,MAOB,CYBB,FMO3,AOX4,ETFA,GCDHB,DLDH,D2HGDH,IVD |
| electron carrier activity | PRKRA,CYP1A2,ACOX3,FDX1L,ACADSB,GCDHB,GCDH,FDX1,COX7A2A,ACADS |
Interacting Protein
GSR has direct interactions with proteins and molecules. Those interactions were detected by several methods such as yeast two hybrid, co-IP, pull-down and so on. We selected proteins and molecules interacted with GSR here. Most of them are supplied by our site. Hope this information will be useful for your research of GSR.
RB1;rhtB3;PJA1;C11orf49
Resources
Related Services
Related Products
References
- Zwiech, R; et al. Absence of C4d urinary excretion in the early post-transplant period is associated with improved long-term kidney graft survival. TRANSPLANT IMMUNOLOGY 30:7-11(2014).
- Gao, C; Huan, J; et al. SDF-1 plays a key role in chronic allograft nephropathy in rats. TRANSPLANTATION PROCEEDINGS 40:1674-1678(2008).


