FGG
-
Official Full Name
fibrinogen gamma chain -
Overview
The protein encoded by this gene is the gamma component of fibrinogen, a blood-borne glycoprotein comprised of three;pairs of nonidentical polypeptide chains. Following vascular injury, fibrinogen is cleaved by thrombin to form fibrin;which is the most abundant component of blood clots. In addition, various cleavage products of fibrinogen and fibrin;regulate cell adhesion and spreading, display vasoconstrictor and chemotactic activities, and are mitogens for several;cell types. Mutations in this gene lead to several disorders, including dysfibrinogenemia, hypofibrinogenemia and;thrombophilia. Alternative splicing results in two transcript variants encoding different isoforms. -
Synonyms
FGG;fibrinogen gamma chain;fibrinogen, gamma polypeptide
Recombinant Proteins
- Human
- Zebrafish
- Rat
- Chicken
- Bovine
- Dog
- Pig
- Sheep
- Rabbit
- Mouse
- E.coli
- HEK293
- Mammalian Cells
- Yeast
- Bovine Plasma
- Dog Plasma
- Porcine plasma
- Sheep Plasma
- Rabbit Plasma
- Rat plasma
- In Vitro Cell Free System
- Human Plasma
- His
- Non
- Avi
- Fc
- DDK
- Myc
- GST
| Cat.# | Product name | Source (Host) | Species | Tag | Protein Length | Price |
|---|---|---|---|---|---|---|
| FGG-12884H | Recombinant Human FGG, His-tagged | E.coli | Human | His | C-term-348a.a. | |
| FGG-103H | Recombinant Human FGG, His-tagged | HEK293 | Human | His | 27-130 a.a. |
|
| FGG-12289Z | Recombinant Zebrafish FGG | Mammalian Cells | Zebrafish | His |
|
|
| FGG-2342R | Recombinant Rat FGG Protein | Mammalian Cells | Rat | His |
|
|
| FGG-438H | Recombinant Human FGG protein, His-tagged | Yeast | Human | His | Lys166~Asn416 |
|
| FGG-6547C | Recombinant Chicken FGG | Mammalian Cells | Chicken | His |
|
|
| FGG-6231HCL | Recombinant Human FGG 293 Cell Lysate | HEK293 | Human | Non |
|
|
| FGG -32B | Native Bovine Fibrinogen | Bovine Plasma | Bovine | Non |
|
|
| FGG -36D | Native Canine Fibrinogen | Dog Plasma | Dog | Non |
|
|
| FGG -41B | Native Bovine Fibrinogen, FITC Labeled | Bovine Plasma | Bovine | Non |
|
|
| FGG -44D | Native Canine Fibrinogen, FITC Labeled | Dog Plasma | Dog | Non |
|
|
| FGG -47P | Native Porcine Fibrinogen, FITC Labeled | Porcine plasma | Pig | Non |
|
|
| FGG -48S | Native Sheep Fibrinogen, FITC Labeled | Sheep Plasma | Sheep | Non |
|
|
| FGG -57R | Native Rabbit Fibrinogen, FITC Labeled | Rabbit Plasma | Rabbit | Non |
|
|
| FGG -60R | Native Rabbit Fibrinogen | Rabbit Plasma | Rabbit | Non |
|
|
| Fgg -65R | Native Rat Fibrinogen, FITC Labeled | Rat plasma | Rat | Non |
|
|
| Fgg -69R | Native Rat Fibrinogen | Rat plasma | Rat | Non |
|
|
| FGG-01H | Recombinant Human FGG Protein (27-437aa), N-His tagged | E.coli | Human | His | 27-437aa |
|
| FGG-1998R | Recombinant Rat FGG Protein, His (Fc)-Avi-tagged | HEK293 | Rat | Avi&Fc&His |
|
|
| FGG-1998R-B | Recombinant Rat FGG Protein Pre-coupled Magnetic Beads | HEK293 | Rat |
|
||
| FGG-2239H | Recombinant Human FGG Protein, MYC/DDK-tagged | HEK293 | Human | DDK&Myc |
|
|
| FGG-2918H | Recombinant Human FGG protein, His&Myc-tagged | E.coli | Human | His&Myc | 27-453aa |
|
| Fgg-3005M | Recombinant Mouse Fgg Protein, Myc/DDK-tagged | HEK293 | Mouse | DDK&Myc |
|
|
| FGG-378H | Recombinant Human FGG protein(Val169-Leu453) | Yeast | Human | Non | Val169-Leu453 |
|
| FGG-427H | Recombinant Human FGG Protein, MYC/DDK-tagged, C13 and N15-labeled | HEK293 | Human | DDK&Myc |
|
|
| FGG-4930HF | Recombinant Full Length Human FGG Protein, GST-tagged | In Vitro Cell Free System | Human | GST | Full L. 453 amino acids |
|
| FGG-53S | Native Sheep Fibrinogen | Sheep Plasma | Sheep | Non |
|
|
| FGG-729B | Recombinant Bovine FGG Protein, His-tagged | E.coli | Bovine | His | Lys168~Asp444 |
|
| FGG-730C | Recombinant Chicken FGG Protein, His-tagged | E.coli | Chicken | His | Tyr27~Ser435 |
|
| FGG-731H | Recombinant Human FGG Protein, His-tagged | E.coli | Human | His | Lys166~Asn416 |
|
| Fgg-732M | Recombinant Mouse Fgg Protein, His-tagged | E.coli | Mouse | His | Val168~Lys405 |
|
| Fgg-733R | Recombinant Rat Fgg Protein, His-tagged | E.coli | Rat | His | Arg170~Gly429 |
|
| FGG-7H | Native Human Fibrinogen, FITC Labeled | Human Plasma | Human | Non |
|
|
| FGG-884M | Recombinant Mouse FGG Protein (Val168-Val436) | Yeast | Mouse | Non | Val168-Val436 |
|
Background
What is FGG protein?
Fibrinogen Gamma Chain (FGG) gene, encoding the FGG protein. This protein plays a pivotal role in the body's clotting cascade, crucial for maintaining hemostasis, an integral event for wound healing.
Discovered through decades of research into the human body's complex web of blood coagulation pathways, the FGG protein is a component of a larger protein structure known as fibrinogen. It was revealed through successive gene mapping and characterization studies, primarily in the latter half of the 20th century.
What Is The Structure of FGG Protein?
The FGG gene occupies the locus 4q31.3 on chromosome 4 in the human genome. Comprising 10 exons and nine introns, this gene spans approximately 8.5 kb. Translated into a protein structure, FGG has a complex quaternary structure with multiple domains, comprised of approximatively 437 amino acids. An understanding of the FGG protein's complex structure is the beginning of comprehending the vast scope of its function and its impact on human health.
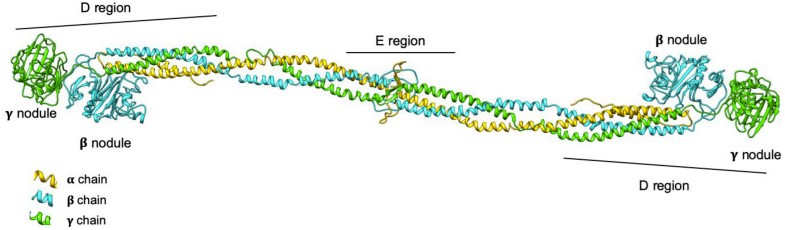
Fig1. 3D model representation of the fibrinogen protein. (Asselta R, et al. 2020)
What Is The Function of FGG Protein?
As already mentioned, the critical function of the FGG protein is in blood coagulation and clot formation, two pivotal processes for wound healing that help maintain a stable internal environment in the body. More specifically, the FGG protein combines with two other peptide chains - alpha (Fibrinogen Alpha Chain/FAG) and beta (Fibrinogen Beta Chain/FBG) - to constitute the hexameric protein fibrinogen. Upon injury to a blood vessel, fibrinogen is converted into fibrin under the influence of a catalyst, thrombin. The fibrin threads then entangle, forming a clot and essentially plugging the wound, preventing further blood loss. The FGG is crucial in this event, with its essential role within the fibrinogen structure and its interactions with other clotting factors and platelets.
The FGG protein is also involved in the regulation of the fibrinolytic system's activity, a series of events to break down formed clots. It is, therefore, a regulator protein interacting within a signal pathway involved in both coagulation and fibrinolysis.
Given the FGG protein's broad involvement in coagulation, any aberrations within its structure or function are potentially detrimental. Mutations in the FGG gene, causing either FGG protein deficiency or dysfunction, are implicated in various diseases. The most common association is with congenital dysfibrinogenemia and hypofibrinogenemia, disorders of fibrinogen production. These conditions may present as bleeding disorders, thromboembolic diseases, or may remain asymptomatic until a triggering event occurs.
Furthermore, research suggests an association of FGG gene variations with the risk of deep venous thrombosis, early-onset ischemic stroke, and potentially, certain pregnancy complications such as preeclampsia. Interestingly, higher FGG-concentrations have also been detected in various malignancies, including colorectal cancer, suggesting a potential role in cancer biology.
FGG Protein's Applications
The importance of FGG protein extends beyond the realm of pathology into practical applications. In diagnostics, differential levels of circulating FGG can indicate various diseases. It may also help monitor the efficacy of anticoagulation therapy or predict the risk of thrombotic events. In therapeutics, synthetic analogs of the FGG protein could potentially serve as part of designer anticoagulant drugs. Gene therapy has also glimpsed at potential strategies for regulating FGG expression in cases of genetic bleeding disorders.
In summary, the FGG protein, encoded by the FGG gene located at 4q31.3, plays an instrumental role in blood coagulation and fibrinolytic pathways in human physiology. Its importance is underscored by the debilitating diseases that arise from FGG gene mutations with ensuing protein deficiency or dysfunction. The vast field of FGG protein research paves the way to further understanding its function and interaction with other biological proteins, thus informing future diagnostic and therapeutic strategies.
Case Study
Case 1: Asselta R, et al. Int J Mol Sci. 2020.
Fibrinogen is a 340-kDa plasma glycoprotein constituted by two sets of symmetrical trimers, each formed by the Aα, Bβ, and γ chains (respectively coded by the FGA, FGB, and FGG genes). Quantitative fibrinogen deficiencies (hypofibrinogenemia, afibrinogenemia) are rare congenital disorders characterized by low or unmeasurable plasma fibrinogen antigen levels. In this article, the authors will briefly review the clinic characteristics of HHHS patients and the histological feature of their hepatic inclusions, and we will focus on the molecular genetic basis of this peculiar type of coagulopathy.

Fig1. Localization and conservation of HHHS -causing mutations in the fibrinogen γ nodule. (a) The localization of all the mutations responsible for the HHHS phenotype, described in the literature, are indicated as grey spheres (in case of missense mutations), and orange spheres (in case of deletion). Mutations are numbered on the mature protein. The fibrinogen chains (Aα, Bβ, γ) are represented in yellow, cyan, and green, respectively. The model was obtained using the pdb structure 3GHG, and the UCSF Chimera package. (b) Multiple alignments of the fibrinogen γ chain terminal regions of different species. Sequences were retrieved from NCBI, and aligned using clustalW. The last line of the alignment shows in a schematic way the conservation among species.
Case 2: Liu YL, et al. Am J Cancer Res. 2020.
Chemotherapy resistance is a major challenge for breast cancer treatment. It is necessary to elucidate the mechanisms of anthracycline resistance to develop new chemosensitizers for breast cancer. In this study, clinical significance of fibrinogen gamma chain (FGG) expression was also analyzed in breast cancer tissues. The researchers provided evidence that breast tumor cell derived FGG participated in anthracycline chemoresistance of breast cancer.
Clinical analysis demonstrated that patients with FGG expressing breast cancer showed a dramatically low response to anthracycline-based chemotherapy and poor survival. TMP was a novel chemosensitizer for FGG-induced anthracycline chemoresistance in breast cancer treatment.
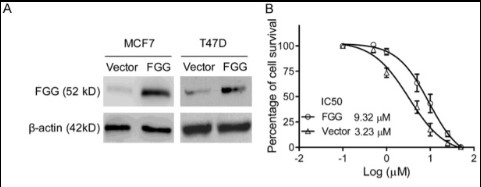
Fig1. FGG promotes breast cancer cell survival and proliferation with anthracycline treatment in vitro and in vivo. A. FGG overexpression was performed with MCF7 and T47D cells. FGG protein levels of infected human breast cancer cells were measured with Western blot assays. β-actin was used as loading control. B. MCF7-FGG/-vector cells were treated with a gradient concentration of EPI.

Fig2. Representative images of clone formation of infected MCF7 and T47D cells, which were captured after 14 days culture.
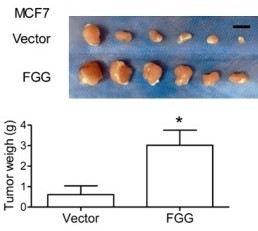
Fig3. Xenografts were harvested and imagined after 15 days treatment. Bar = 1 cm. The tumor weights were compared between two groups. Data represent the means ± SD. *P < 0.05.
Case 3: Peng HH, et al. Front Genet. 2021.
Castration-resistant prostate cancer (CRPC) threatens the health of men in general and no effective therapeutics currently exists for the treatment of CRPC. It is therefore of great importance to find a novel molecule that can be a biomarker and a therapeutic target for CRPC.
The authors found that the serum fibrinogen gamma (FGG) levels in patients with CRPC were significantly higher than those with localized prostate cancer. And they established that FGG is modulated by IL-6 which was increased in CRPC patients via phosphorylation of STAT3. The data suggests that FGG may be a potential therapeutic target and prognostic marker for CRPC.
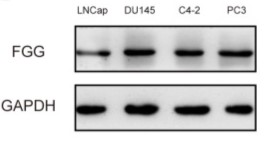
Fig1. Relative FGG protein expression in PCa cell lines.

Fig2. Colony formation assays shows that FGG knockdown significantly decreased cell proliferation in PC3 and DU145 cells.
Quality Guarantee
Involved Pathway
FGG involved in several pathways and played different roles in them. We selected most pathways FGG participated on our site, such as Complement and coagulation cascades,Platelet activation,Staphylococcus aureus infection, which may be useful for your reference. Also, other proteins which involved in the same pathway with FGG were listed below. Creative BioMart supplied nearly all the proteins listed, you can search them on our site.
| Pathway Name | Pathway Related Protein |
|---|---|
| Staphylococcus aureus infection | ITGAM,HLA-DQB1,C3AR1,CFI,PTAFR,C1S,HLA-DRB5,HLA-DPB1,FPR1,DSG1A |
| Complement and coagulation cascades | F3,FGA,F2R,C1QB,MASP1,C4A,LOC100514666,KNG1,PLG,C2 |
| Platelet activation | ARHGAP35,FYN,STIM1,GUCY1B3,APBB1IP,Adcy4,MYL12B,MAPK3,ITGA2B,GNAQ |
Protein Function
FGG has several biochemical functions, for example, cell adhesion molecule binding,metal ion binding,protein binding, bridging. Some of the functions are cooperated with other proteins, some of the functions could acted by FGG itself. We selected most functions FGG had, and list some proteins which have the same functions with FGG. You can find most of the proteins on our site.
| Function | Related Protein |
|---|---|
| cell adhesion molecule binding | CD1d1,NLGN2,CADM2A,EZR,PVRL2,POSTNB,CD226,PTPRD,CAML,PVRL4 |
| structural molecule activity | KRT95,LAMA2,CLDND,MAP4,SEPT7,MAP7D2A,VPS25,KRT7,KRT32,KRT1-C5 |
| metal ion binding | CEPT1,B4GALT3,ZNF434,ZSCAN21,YME1L1,ANKIB1,ZBTB2,ZNF71,STAMBP,CDC7 |
| protein binding, bridging | LOR,ANK2,NEFH,SPRR1B,TRIM17,TNNT2A,SPRR1A,CBX5,DSP,ANXA1 |
| receptor binding | HCK,CNPY3,CRTAM,SMARCD3,PCSK1N,CD70,CNPY4,C1QTNF2,CADM1,Ifnl2 |
Interacting Protein
FGG has direct interactions with proteins and molecules. Those interactions were detected by several methods such as yeast two hybrid, co-IP, pull-down and so on. We selected proteins and molecules interacted with FGG here. Most of them are supplied by our site. Hope this information will be useful for your research of FGG.
FGA;ICAM1;q9wmx2-pro_0000037550;d-mannose
Resources
Research Area
Related Services
Related Products
References
- You, QM; Verschoor, CP; et al. Proteomic analysis of plasma from Holstein cows testing positive for mycobacterium avium subsp Paratuberculosis (MAP). VETERINARY IMMUNOLOGY AND IMMUNOPATHOLOGY 148:243-251(2012).
- Kaiser, R; Li, YH; et al. Genetic Risk Factors for Thrombosis in Systemic Lupus Erythematosus. JOURNAL OF RHEUMATOLOGY 39:1603-1610(2012).







