COMP
-
Official Full Name
cartilage oligomeric matrix protein -
Overview
Cartilage oligomeric matrix protein is a protein that in humans is encoded by the COMP gene. The protein encoded by this gene is a noncollagenous extracellular matrix (ECM) protein. It consists of five identical glycoprotein subunits, each with EGF-like and calcium-binding (thrombospondin-like) domains. Oligomerization results from formation of a five-stranded coiled coil and disulfide bonds. Binding to other ECM proteins such as collagen appears to depend on divalent cations. Mutations can cause the osteochondrodysplasias pseudoachondroplasia (PSACH) and multiple epiphyseal dysplasia (MED) -
Synonyms
COMP;cartilage oligomeric matrix protein;cartilage oligomeric matrix protein (pseudoachondroplasia, epiphyseal dysplasia 1, multiple) , EDM1, EPD1, PSACH;MED;THBS5;thrombospondin 5;TSP5;thrombospondin-5;pseudoachondroplasia (epiphyseal dysplasia 1
Recombinant Proteins
- Human
- Bacillus subtilis
- Rat
- Mouse
- E.coli
- HEK293
- E.Coli/Yeast
- Mammalian Cells
- Wheat Germ
- CHO
- Human Cells
- In Vitro Cell Free System
- GST
- His
- Flag
- T7
- Non
- Avi
- Fc
- DDK
- Myc
Background
What is COMP Protein?
COMP gene (cartilage oligomeric matrix protein) is a protein coding gene which situated on the short arm of chromosome 19 at locus 19p13. The protein encoded by this gene is a non-collagenous extracellular matrix (ECM) protein consisting of five identical glycoprotein subunits, each with an EGF-like portion and a calcium-binding domain. The five subunits are bound together by forming five strands of coil and disulfide. Its binding to other ECM proteins, such as collagen, seems to depend on bivalent cations. Contraction or expansion of the 5-amino acid aspartic acid repeat sequence, as well as other mutations, can lead to pseudochondroplasia (PSACH) and multiple epiphyseal dysplasia (MED). The COMP protein is consisted of 757 amino acids and COMP molecular weight is approximately 82.9 kDa.
What is the Function of COMP Protein?
COMP protein, or Cartilage Oligomeric Matrix Protein, plays a critical role in the structure and function of the extracellular matrix in cartilage. It's made up of five identical subunits, each featuring EGF-like and calcium-binding domains, that come together to form a strong coiled coil structure. This protein mainly interacts with other matrix proteins, like collagen, through divalent cations. It's important for maintaining cartilage integrity, and any disruptions in its structure, like a change in the aspartate repeat sequence or other mutations, can lead to disorders like pseudochondroplasia (PSACH) and multiple epiphyseal dysplasia (MED).
COMP Related Signaling Pathway
Based on existing knowledge, COMP, or Cartilage Oligomeric Matrix Protein, plays a critical role in maintaining cartilage stability and integrity. It's heavily involved in bone development and repair processes. When it comes to its signaling pathways, COMP interacts with various molecules within the extracellular matrix, impacting chondrocyte behavior and cartilage formation. Aberrations in these pathways, due to mutations or other factors, can lead to conditions like pseudoachondroplasia and multiple epiphyseal dysplasia, which affect bone growth and structure. If you have access to articles or need insights on a specific pathway, it might be helpful to check scientific databases or journals for detailed research findings.
COMP Related Diseases
COMP (Cartilage Oligomeric Matrix Protein) is linked to several genetic disorders, prominently including pseudoachondroplasia (PSACH) and multiple epiphyseal dysplasia (MED). These are skeletal dysplasias resulting from mutations in the COMP gene. In these conditions, the malformation and abnormal growth of bones particularly affect the long bones and spinal column. Cartilage anomalies and early onset arthritis are common clinical presentations. COMP-related disorders impact the assembly and maintenance of the cartilage matrix, leading to the structural and functional defects characteristic of these diseases. Understanding and researching these connections further our grasp of skeletal disease mechanisms, with direct implications for diagnosis and targeted therapies.
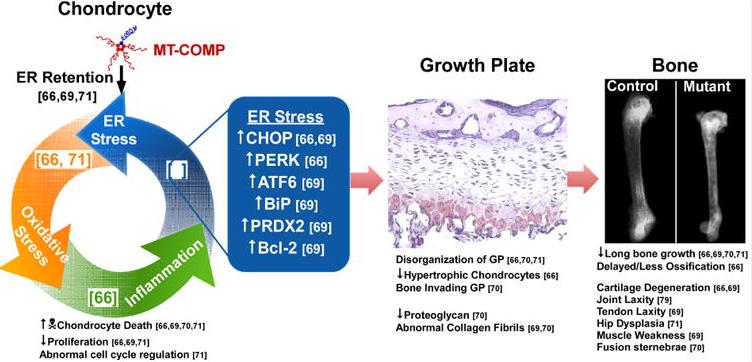
Fig1. Pathology associated with mutant COMP. (Karen L Posey, 2019)
Bioapplications of COMP
COMP is often used in biomedical research to study bone and joint health because it plays an important function in cartilage tissue. It helps maintain the structural integrity of cartilage and has received a lot of attention in the study of diseases such as arthritis. In addition, the expression level of COMP can be used as a marker for some bone and joint diseases, so as to be used for disease diagnosis and progression detection. Although current research has focused on its role in bone diseases, COMP may show potential in more biomedical applications as the technology advances.
Case Study
Case Study 1: Alvaro H Crevenna, 2016
We already know the sorting and transport process of transmembrane proteins and lysosomal hydrolases in the Golgi network (TGN), but it is still unclear how secretory cargoes are separated and released outside the cell. Previous studies have shown that the soluble protein Cab45 in the TGN is required for the sorting of secretory cargoes in the presence of calcium ions (Ca²⁺). It was further discovered that Cab45 aggregates into oligomers in the presence of Ca²⁺ and specifically binds to secretory proteins such as COMP and LyzC. In cells, if the calcium binding site of Cab45 mutates, its aggregation ability and sorting function for COMP and LyzC will be impaired. Super-resolution microscopy also showed that Cab45 co-localizes with secretory proteins and the TGN calcium pump (SPCA1) in specific TGN microregions.
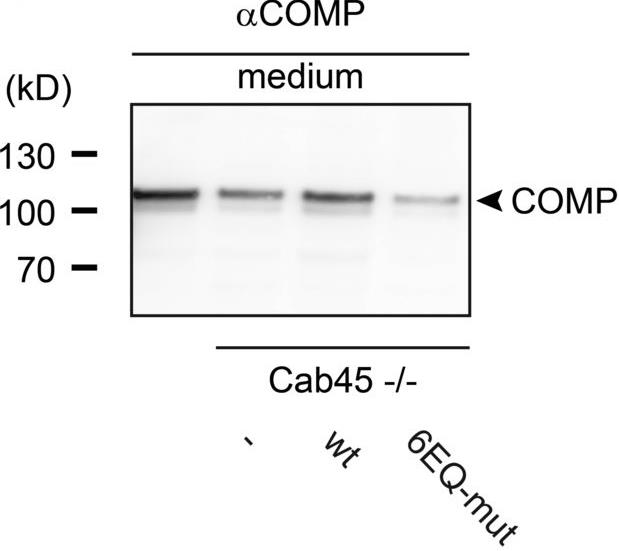
Fig1. Media and cell extracts were blotted and probed with antibodies against COMP.
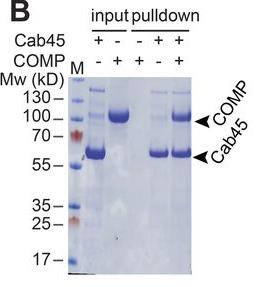
Fig2. Pull-down experiments performed with His-tagged Cab45 and the potential cargo molecules COMP.
Case Study 2: Matthew J. Rock, 2010
Cartilage oligomeric matrix protein (COMP) is basically a big protein that hangs out in places like cartilage, tendons, and ligaments. It's pretty crucial for understanding conditions like pseudoachondroplasia (PSACH) and multiple epiphyseal dysplasia (MED), where you get issues like short stature or early arthritis. Researchers looked into how COMP works with ligament cells and found that cells stick to COMP better when there's more of it around, but not if you block it with a specific peptide. They figured out that a couple of molecules on the cell surface, CD47 and αVβ3 integrin, are key to this sticking action. When cells latch onto COMP, they end up sprouting little actin spikes, thanks to a protein called fascin. So, COMP is kind of like a double agent, getting cells to attach through CD47 and integrin and then jazzing them up with fascin-actin spikes.
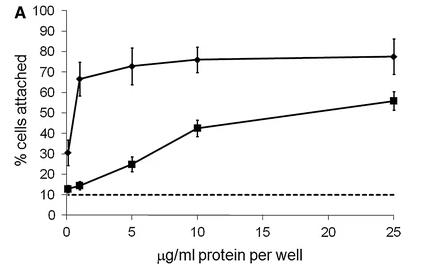
Fig3. Ligament cells were plated on wells coated with recombinant COMP and Cell adhesion is expressed.
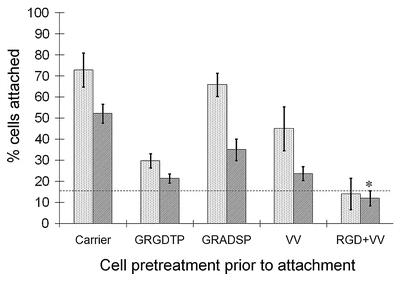
Fig4. Ligament cell attachment to COMP is independently inhibited by either the VV-containing peptide or an RGD peptide.
Quality Guarantee
High Purity
.jpg)
Fig1. SDS-PAGE (COMP-5794H)
.
.jpg)
Fig2. SDS-PAGE (COMP-675H)
Involved Pathway
COMP involved in several pathways and played different roles in them. We selected most pathways COMP participated on our site, such as ECM proteoglycans,ECM-receptor interaction,Extracellular matrix organization, which may be useful for your reference. Also, other proteins which involved in the same pathway with COMP were listed below. Creative BioMart supplied nearly all the proteins listed, you can search them on our site.
| Pathway Name | Pathway Related Protein |
|---|---|
| ECM-receptor interaction | ITGB1B.2,ITGB1A,DAG1,COL4A4,COL4A5,LAMA1,COL4A1,THBS4A,SV2A,COL6A3 |
| Focal adhesion | Reln,PARVA,FLNB,PIP5K1C,SHC1,ITGB8,HGF,COL11A1A,PRKCA,BIRC2 |
| Integrin cell surface interactions | COL16A1,ICAM4,JAM2B,COL13A1,COL23A1,LOC100514666,FN1B,F11R.2,FBN1,COL9A1B |
| Malaria | CD36,GYPB,IFNG,SELP,GYPC,VCAM1,HBA2,SELE,TGFB1,ITGAL |
| Extracellular matrix organization | COL17A1,JAM2B,COL5A2,MGC174857,MMP10,MFAP1B,COL3A1,MFAP4,LOX,COL28A1A |
| PI3K-Akt signaling pathway | CSH1,EFNA3,CSF3,LOC608458,CSH2,PIK3AP1,CHAD,EFNA4,PRP2,CSF1 |
| ECM proteoglycans | VCANB,COL9A1,MATN1,COL9A3,DMP1,BGNA,ITGB3A,DAG1,FMOD,LRP4 |
Protein Function
COMP has several biochemical functions, for example, calcium ion binding,collagen binding,extracellular matrix structural constituent. Some of the functions are cooperated with other proteins, some of the functions could acted by COMP itself. We selected most functions COMP had, and list some proteins which have the same functions with COMP. You can find most of the proteins on our site.
| Function | Related Protein |
|---|---|
| heparan sulfate proteoglycan binding | GPC3,GPC6A,AGRN,GPC6,SLIT2,HPSE2,SEMA5A,Itgam&Itgb2,GPC4,UBE4A |
| protein binding | WASH1,PABPC4,KIAA0947,RGS6,ATXN7L1,IGFBP7,COPS7A,AVPR1A,AKAP1,SGPL1 |
| protease binding | FLOT2,INS1,TRIP4,CCDC71L,CFLAR,SERPINB4,ADAMTS4,DERL1,RNF139,CSTA |
| extracellular matrix structural constituent | COL1A1A,COL27A1,COL3A1,COL1A2,CHI3L1,LUM,COL11A2,BGN,ACAN,PXDN |
| heparin binding | CXCL13,RPL29,EPYC,CXCL11,PRELP,RSPO4,FGFRL1,PDCD5,CCL5,NRP1A |
| collagen binding | ITGA2,ASPN,ITGA9,ANTXR1,SERPINH2,GPR56,RELL2,LRRC15,LUM,PAK1 |
| calcium ion binding | HMCN1,S100A2,S100A13,PLCZ1,CCDC48,NUCB2B,FAT4,PLCG1,BGLAP2,PCDHGC3 |
Interacting Protein
COMP has direct interactions with proteins and molecules. Those interactions were detected by several methods such as yeast two hybrid, co-IP, pull-down and so on. We selected proteins and molecules interacted with COMP here. Most of them are supplied by our site. Hope this information will be useful for your research of COMP.
ADAMTS12;OTX1;MATN3;ACAN;ADAMTS7
Resources
Related Services
Related Products
References
- Butz, E; Peichl, L; et al. Cone Bipolar Cells in the Retina of the Microbat Carollia perspicillata. JOURNAL OF COMPARATIVE NEUROLOGY 523:963-981(2015).
- Stanton, GB; Kohler, SJ; et al. Cytogenesis in the Adult Monkey Motor Cortex: Perivascular NG2 Cells Are the Major Adult Born Cell Type. JOURNAL OF COMPARATIVE NEUROLOGY 523:849-868(2015).



