Capn2
-
Official Full Name
calpain 2, (m/II) large subunit -
Overview
The calpains, calcium-activated neutral proteases, are nonlysosomal, intracellular cysteine proteases. The mammalian calpains include ubiquitous, stomach-specific, and muscle-specific proteins. The ubiquitous enzymes consist of heterodimers with distinct large, catalytic subunits associated with a common small, regulatory subunit. This gene encodes the large subunit of the ubiquitous enzyme, calpain 2. Multiple heterogeneous transcriptional start sites in the 5 UTR have been reported. Two transcript variants encoding different isoforms have been found for this gene. [provided by RefSeq, Mar 2009] -
Synonyms
CAPN2;calpain 2, (m/II) large subunit;CANP2;mCANP;CANPL2;CANPml;calpain-2 catalytic subunit;CANP 2;M-calpain;calpain M-type;millimolar-calpain;calpain-2 large subunit;calpain 2, large subunit;calpain large polypeptide L2;calpain, large polypeptide L2;calpain 2, large [catalytic] subunit;calcium-activated neutral proteinase 2
Recombinant Proteins
- Human
- Rat
- Cynomolgus
- Porcine
- Mouse
- Rhesus macaque
- Chicken
- Pig
- Bovine
- E.coli
- Mammalian Cells
- Porcine kidney
- Wheat Germ
- HEK293
- HeLa
- Bovine Myocardium
- In Vitro Cell Free System
- GST
- His
- Non
- DDK
- Myc
- Avi
- Fc
- Flag
Background
What is CAPN2 protein?
CAPN2 (calpain 2) gene is a protein coding gene which situated on the long arm of chromosome 1 at locus 1q41. The calpains, calcium-activated neutral proteases, are nonlysosomal, intracellular cysteine proteases. The mammalian calpains include ubiquitous, stomach-specific, and muscle-specific proteins. The ubiquitous enzymes consist of heterodimers with distinct large, catalytic subunits associated with a common small, regulatory subunit. This gene encodes the large subunit of the ubiquitous enzyme, calpain 2. Multiple heterogeneous transcriptional start sites in the 5' UTR have been reported. The CAPN2 protein is consisted of 700 amino acids and its molecular mass is approximately 80.0 kDa.
What is the function of CAPN2 protein?
Survival and apoptosis: CAPN2 is involved in the regulation of cell survival and apoptosis, and its abnormal activation is associated with the occurrence and development of many diseases. CAPN2 is involved in the degradation of muscle protein and is associated with muscle growth and maintenance of muscle function. The expression of CAPN2 regulates the migration and invasion of pancreatic cancer cells, possibly by affecting the epithelial-mesenchymal transition (EMT) and Wnt/β-catenin signaling pathways. CAPN2 is related to autophagy, and its abnormal expression may affect autophagy and lysosomal function. CAPN2 is involved in the regulation of cell cycle, and the change of its expression level may affect the process of cell cycle.
CAPN2 Related Signaling Pathway
In non-small cell lung cancer, CAPN2 promotes drug resistance, proliferation, and apoptosis inhibition via the EGFR (epidermal growth factor receptor) /AKT/survivin signaling pathway. CAPN2 regulates epithelial-mesenchymal transformation (EMT) and tumor cell migration and invasion through the Wnt/β-catenin signaling pathway in pancreatic cancer. CAPN2 is involved in cytoskeletal protein remodeling and influences cell morphology and movement. CAPN2 plays a role in apoptosis, regulating cell survival or death by cutting a variety of substrate proteins. In some tumors, CAPN2 may be involved in regulating tumor angiogenesis, affecting tumor blood supply and growth.
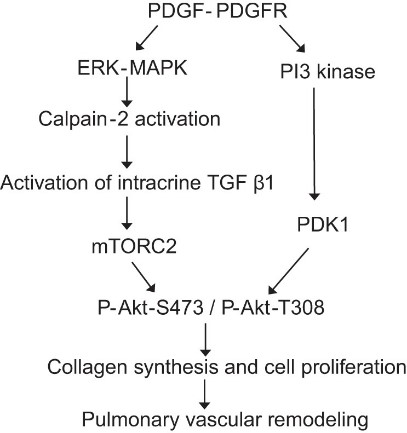
Fig1. Schematic pathway illustrating the role of calpain-2 in PDGF-induced Akt phosphorylation in PASMCs of pulmonary vascular remodeling. (Prasanna Abeyrathna, 2016)
CAPN2 Related Diseases
The CAPN2 protein is associated with a variety of diseases, especially tumors, neurological diseases, and muscle diseases. In terms of tumor, CAPN2 is associated with drug resistance of non-small cell lung cancer, the occurrence and development of breast cancer and ovarian cancer. In neurological diseases, CAPN2 has been associated with major depression, stroke (including hemorrhagic and ischemic), Parkinson's disease, schizophrenia, and spinocerebellar ataxia type 3. In muscle diseases, CAPN2 is associated with muscular dystrophy. In addition, CAPN2 is also associated with idiopathic pulmonary fibrosis, malaria, diabetic retinopathy, glaucoma, pulmonary hypertension, chronic lymphocytic leukemia, etc. In these diseases, CAPN2 may play a role by affecting cell signaling, cell cycle, apoptosis and other processes.
Bioapplications of CAPN2
CAPN2 has been found to be overexpressed in a variety of cancers and is associated with tumor proliferation, invasion, and chemotherapy resistance. For example, in hepatocellular carcinoma (HCC), CAPN2 forms a positive feedback loop with SRC kinase that promotes beta-catenin accumulation and signal activation, thereby driving tumor progression. In non-small cell lung cancer (NSCLC), CAPN2 knockdown promotes apoptosis and restores sensitivity to gefitinib via the epidermal growth factor receptor/protein kinase B/Survivin signaling pathway. Capn2-responsive mesoporous silicon nanoparticles (MSNs) are being investigated for drug delivery to reduce chemotherapeutic drug-induced leukopenia and do not cause organ damage compared to conventional systemic administration.
Case Study
Case Study 1: Rui Feng, 2020
This study is aimed to investigate whether calpain 2 (CAPN2) serves as an indicator of the hepatitis B virus (HBV) to induce hepatic fibrosis. Differentially-expressed genes (DEGs) in HBV-induced hepatic fibrosis and normal liver tissues were analyzed, and signal pathway which was analyzed by the Kyoto Encyclopedia of Genes and Genomes (KEGG) analysis using DEGs. Next, the gene-related network map was constructed using the Search Tool for the Retrieval of Interacting Genes. Moreover, CAPN2 protein expression, level of hepatic fibrosis, CAPN2 messenger RNA level, and protein levels of CAPN2, a-SAM, COL3A1, COL1A1, and MAPK1 were determined using Immunohistochemistry (IHC), hematoxylin and eosin, RT-qPCR, and western blot (WB), respectively. There were 420 DEGs screened in HBV-induced hepatic fibrosis and normal liver tissues, among which, 373 were significantly upregulated and 47 were obviously downregulated. The network diagram analysis showed that the DEGs, such as CAPN2, ITGAV, and CCR2, play the key role in the DEG network map, and CAPN2 related to hepatic fibrosis via MAPK1. The increased CAPN2 expression and obvious hepatic fibrosis was displayed in the HBV-induced hepatic fibrosis tissues. In addition, HBV could induce CAPN2 expression, and the interference of CAPN2 could inhibit the expression of hepatic fibrosis markers, including a-SAM, COL3A1, COL1A1, and MAPK1. CAPN2 is regarded as a biomarker of hepatic fibrosis induced by HBV.
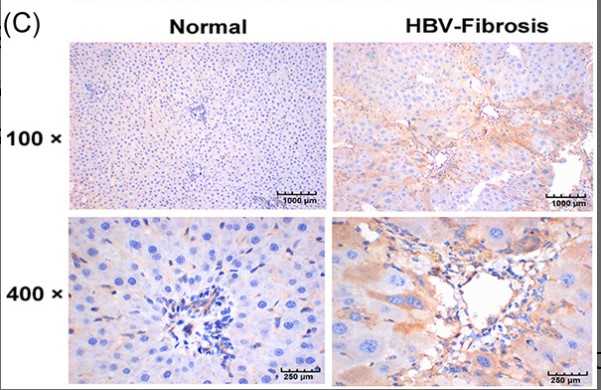
Fig1. Immunohistochemistry detection of CAPN2 protein expression in mouse liver tissues.
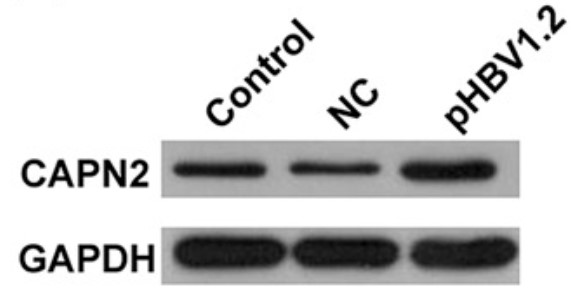
Fig2. CAPN2 protein level in HepG2.
Case Study 2: Makoto Ishihara, 2013
Adult T-cell leukemia (ATL) is one of the most aggressive hematologic malignancies caused by human T-lymphotropic virus type 1 (HTLV-1) infection. The prognosis of ATL is extremely poor; however, effective strategies for diagnosis and treatment have not been established. To identify novel therapeutic targets and diagnostic markers for ATL, the researchers employed focused proteomic profiling of the CD4(+)CD25(+)CCR4(+) T-cell subpopulation in which HTLV-1-infected cells were enriched. Comprehensive quantification of 14 064 peptides and subsequent 2-step statistical analysis using 29 cases identified 91 peptide determinants that statistically classified 4 clinical groups with an accuracy rate of 92.2% by cross-validation test. Among the identified 17 classifier proteins, α-II spectrin was drastically accumulated in infected T cells derived from ATL patients, whereas its digestive protease calpain-2 (CAN2) was significantly downregulated. Further cell cycle analysis and cell growth assay revealed that rescue of CAN2 activity by overexpressing constitutively active CAN2 (Δ(19)CAN2) could induce remarkable cell death on ATL cells accompanied by reduction of α-II spectrin.
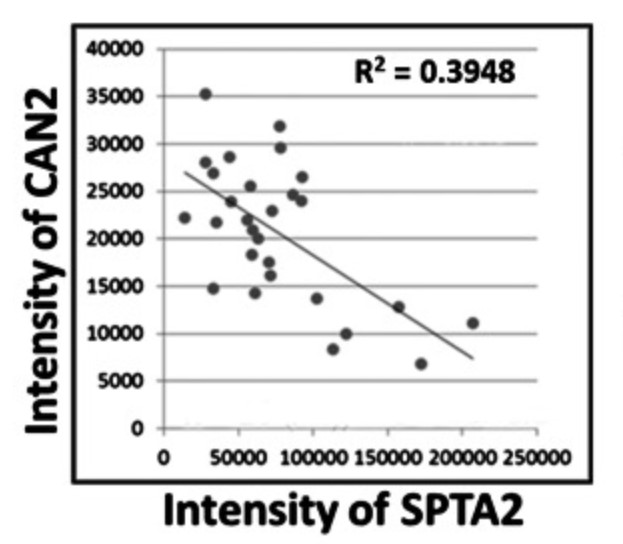
Fig3. Correlation between CAN2 and SPTA2 expression level in 27 cases.
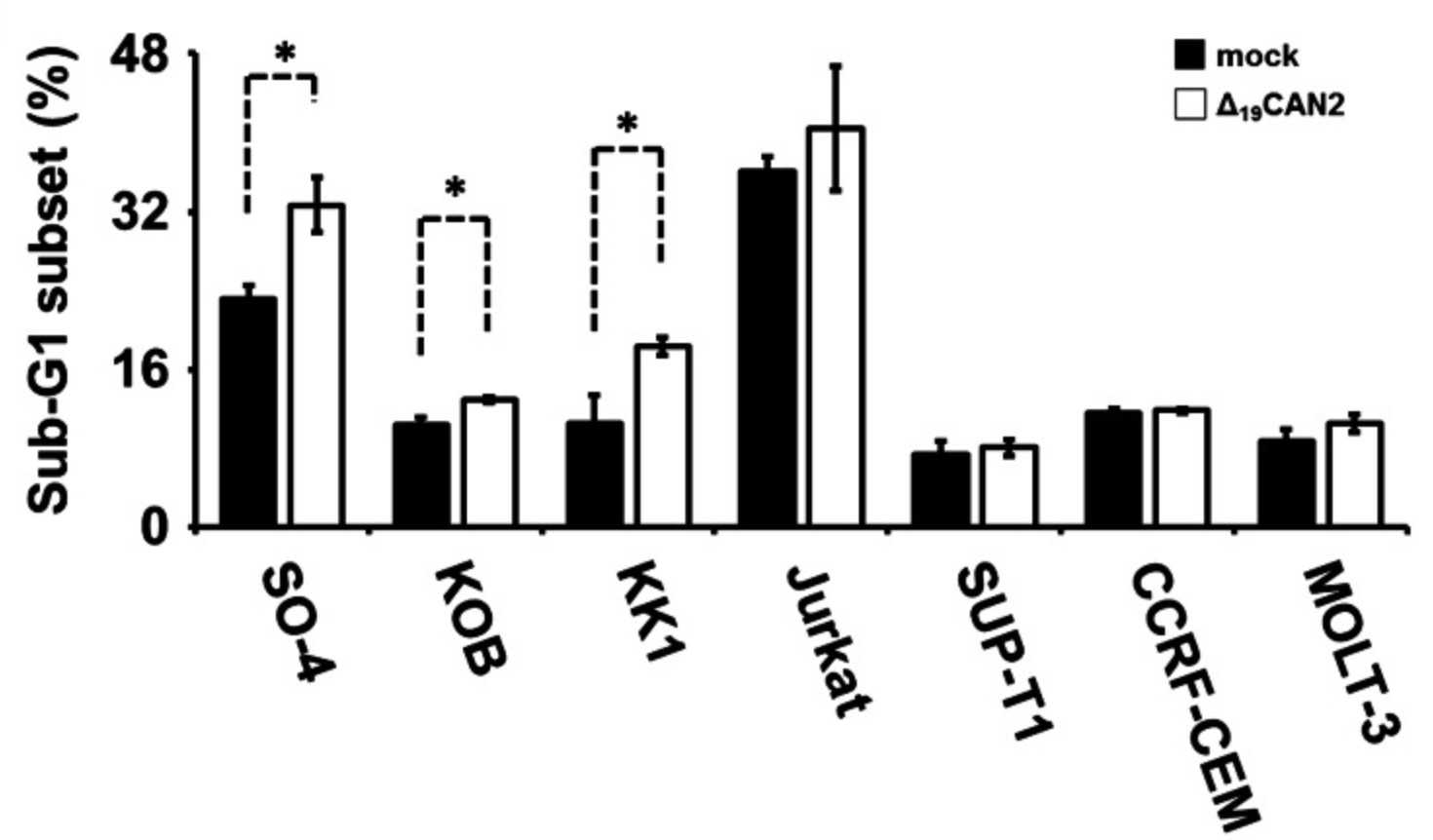
Fig4. Overexpression of Δ19CAN2 significantly accelerated cell death.
Quality Guarantee
High Purity
.jpg)
Fig1. SDS-PAGE (CAPN2-122H)
.
.jpg)
Fig2. SDS-PAGE (CAPN2-0363H)
Involved Pathway
Capn2 involved in several pathways and played different roles in them. We selected most pathways Capn2 participated on our site, such as Protein processing in endoplasmic reticulum,Apoptosis,Focal adhesion, which may be useful for your reference. Also, other proteins which involved in the same pathway with Capn2 were listed below. Creative BioMart supplied nearly all the proteins listed, you can search them on our site.
| Pathway Name | Pathway Related Protein |
|---|---|
| Focal adhesion | IGF1RB,SPP1,RAP1A,HGF,IGF1,PTK2AB,COL2A1B,CAPN2B,FYNA,BCAR1 |
| Alzheimers disease | BACE2,CAPN1,mt-Atp8,NDUFV3,NDUFS2,Casp3,ATP5O,UQCRC1,GSK3B,COX5A |
| Protein processing in endoplasmic reticulum | ERLEC1,SEC63,MAP2K7,HSPA1L,MAPK8B,SEC31A,SSR2,SEC61G,FBXO6,LMAN1L |
| Apoptosis | CASP3A,ENDOG,IL3RA,YWHAQ,DFFB,IL1B,IRAK1,CASP6,Gzmg,FAS |
Protein Function
Capn2 has several biochemical functions, for example, calcium ion binding,calcium-dependent cysteine-type endopeptidase activity,cysteine-type peptidase activity. Some of the functions are cooperated with other proteins, some of the functions could acted by Capn2 itself. We selected most functions Capn2 had, and list some proteins which have the same functions with Capn2. You can find most of the proteins on our site.
| Function | Related Protein |
|---|---|
| cytoskeletal protein binding | ALDOC,EPB41L4B,LDB3,PTPN4,ANXA2,EPB4.1L4A,EPB41B,FARP2,LIN7C,ALDOA |
| cysteine-type peptidase activity | DESI2,CAPN5A,USP43A,PSD,USP9X,CASP6,CAPN2A,CTSW,UFSP2,CAPN2B |
| calcium ion binding | PCDH2G29,EHD1A,PCDH2AB12,DSG1A,CALR,NRXN1,HPCAL1,ACTN2B,CBLB,SLC25A23 |
| protein binding | RITA1,FRMPD4,FAM118A,KRTAP9-2,MGST1,PPP1R8,TIE1,SNRPB2,VPS24,ZNF593 |
| calcium-dependent cysteine-type endopeptidase activity | GCA,CAPN3A,CAPN2B,CAPN13,SOLH,CAPN2L,CAPN1B,CAPN1A,CAPN2A,PEF1 |
| protein heterodimerization activity | MICU1,BIRC5,NPM1,H2AFB2,HMG20A,CD3D,NAE1,H2AFVB,PDSS1,HOMER1 |
Interacting Protein
Capn2 has direct interactions with proteins and molecules. Those interactions were detected by several methods such as yeast two hybrid, co-IP, pull-down and so on. We selected proteins and molecules interacted with Capn2 here. Most of them are supplied by our site. Hope this information will be useful for your research of Capn2.
CAPNS1;ZDHHC17;PTPN1;TNS1;CAST;NMT1;SUMO1;15-deoxy-delta(12,14;JAK3;CHORDC1
Resources
Related Services
Related Products
References




