ATP7B
-
Official Full Name
ATPase, Cu++ transporting, beta polypeptide -
Overview
This gene is a member of the P-type cation transport ATPase family and encodes a protein with several membrane-spanning domains, an ATPase consensus sequence, a hinge domain, a phosphorylation site, and at least 2 putative copper-binding sites. This protein functions as a monomer, exporting copper out of the cells, such as the efflux of hepatic copper into the bile. Alternate transcriptional splice variants, encoding different isoforms with distinct cellular localizations, have been characterized. Mutations in this gene have been associated with Wilson disease (WD). -
Synonyms
ATP7B;ATPase, Cu++ transporting, beta polypeptide;ATPase, Cu++ transporting, beta polypeptide (Wilson disease) , WND;copper-transporting ATPase 2;Wilson disease;copper pump 2;Wilson disease-associated protein;ATPase, Cu(2+)- transporting, beta poly
Recombinant Proteins
- Human
- Mouse
- E.coli
- Wheat Germ
- Mammalian Cells
- HEK293
- His
- GST
- Non
- Flag
- DDK
- Myc
- Avi
- Fc
Background
What is ATP7B Protein?
ATP7B is a protein that's crucial for moving copper in our bodies. It's mostly hanging out in the liver and brain regions, making sure there's a good balance of copper by helping eliminate excess amounts through bile or plasma. Problems with the ATP7B gene can lead to Wilson disease, which can cause copper to build up in the body, potentially leading to liver and brain issues. The protein is made up of several parts, like regions that help it bind ATP and others that help it manage copper intake and export. Overall, ATP7B is like a traffic cop, directing where copper should go and keeping everything flowing smoothly.
What is the Function of ATP7B Protein?
The ATP7B protein, also known as the Wilson disease protein, is a copper-transporting enzyme in our bodies that plays a crucial role in handling copper levels, particularly in the liver and brain. Simply put, ATP7B helps shuttle copper to where it's needed for various enzymatic tasks. It works like a little transporter in the trans-Golgi network, mainly showing up in liver cells. One of its main gigs is making sure there's no copper overload. When everything's in balance, it neatly packages extra copper to be whisked away into bile or sent into the plasma for elimination. But if the ATP7B gene goes awry, it can trigger Wilson's disease, which is basically the body's way of saying there's too much copper hanging around, leading to potential liver hiccups and neurological troubles. Just think of ATP7B as an essential copper janitor, doing its best to keep everything spick and span!
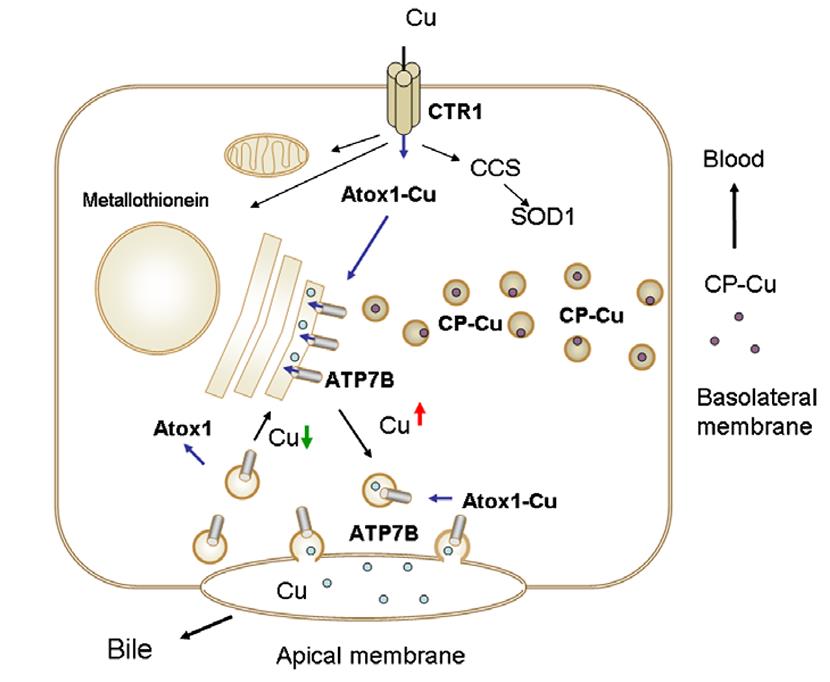
Fig1. The dual role of copper-transporting ATPase ATP7B in hepatocyte. (Svetlana Lutsenko, 2007)
ATP7B Related Signaling Pathway
The ATP7B protein plays a major role in copper management within our bodies. It mainly hangs out in the liver's trans-Golgi network, but you can also find a bit of it in the brain. Basically, it helps get copper to the enzymes in the Golgi apparatus that need it. Think of the liver as the main copper control hub, getting rid of any excess copper. ATP7B jumps into action by shooting copper into the bile or releasing it into the plasma to keep things in balance. It's when the ATP7B gene doesn't quite function right that we start seeing issues like Wilson's disease, where copper builds up and can cause a whole host of problems.
ATP7B Related Diseases
The ATP7B gene is like a key player in managing copper in the body, and when it goes awry, it leads to a condition known as Wilson disease. This gene holds the blueprints for a protein tasked with moving copper, mostly hanging out in the liver but also found in the kidneys and brain. Copper isn't just there for decoration; it's essential for certain enzymes and flushing out excess copper from the body. But when mutations in ATP7B occur, it throws things off balance—more than 250 mutations have been linked to Wilson's disease. These mutations might mess with the protein's structure, or sometimes, they might ensure the protein doesn't form at all. This genetic confusion means copper isn't booted out as it should be, causing it to pile up dangerously in the liver and brain, damaging these organs over time.
Bioapplications of ATP7B
The recombinant ATP7B protein has some interesting roles, especially in the research and industrial production fields. Scientists are quite keen on exploring its use in understanding Wilson's disease, which is all about copper accumulation in the body. By studying a recombinant version of ATP7B, researchers can get into the nitty-gritty of how copper is metabolized, offering insights into new medical treatments for this genetic disorder. In terms of industrial production, ATP7B is handy in biotechnology for creating systems that manage metal ion concentrations, making it valuable in metal detoxification processes. By harnessing its copper-transporting ability, it's possible to enhance the efficacy and efficiency of systems designed to detoxify heavy metals, which is a big win for both scientific research and environmental management.
Case Study
Case Study 1: Shubhrajit Roy, 2020
Wilson disease (WD) is a genetic condition where mutations in the ATP7B gene, responsible for copper transport, cause problems. Many patients actually have different mutations on each allele, but most research has only focused on when both alleles have the same mutation. This study looked at five specific mutations found in Indian WD patients to see how they act alone and together. The mutations A595T, S1362A, and S1426I don't mess with where ATP7B goes inside cells but do decrease its ability to transport copper. Interestingly, S1362A also stops ATP7B from moving when copper levels change. The G1061E and G1101R mutations, found in the ATP-binding area, trap ATP7B in the endoplasmic reticulum, messing with copper movement and reducing ATP7B levels. Together, when A595T and G1061E are both present, they change how ATP7B gets around inside cells depending on the copper levels, making this study relevant for understanding compound-heterozygous states in WD patients.
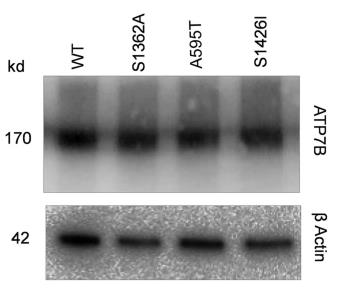
Fig1. Representative Western blot analysis of the WT, S1362A, A595T, and S1426I protein abundances.
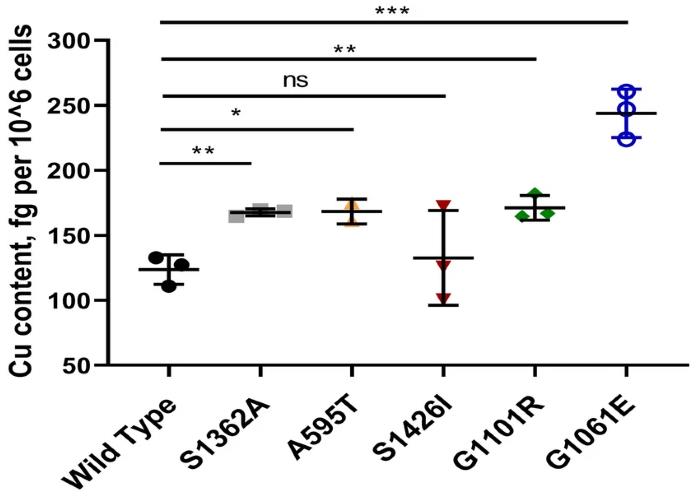
Fig2. Cu retention in YST cells expressing ATP7B mutants.
Case Study 2: Agnese Padula, 2022
Wilson disease (WD) messes up how your body handles copper because of a glitch in the ATP7B transporter gene. Gene therapy using adeno-associated vectors (AAV) seems promising, but the whole ATP7B gene is too big for these vectors. To tackle this, researchers came up with a dual AAV system using split inteins. These unique proteins stitch together two separate protein halves perfectly. They used a special intein from Nostoc punctiforme to stitch together the ATP7B gene. By splitting the ATP7B gene and packaging each half into separate AAVs—with one half also having part of the intein—scientists managed to reassemble the full gene inside liver cells after injecting the vectors into mice. This method worked well, helping WD mice manage copper better and avoid liver troubles.
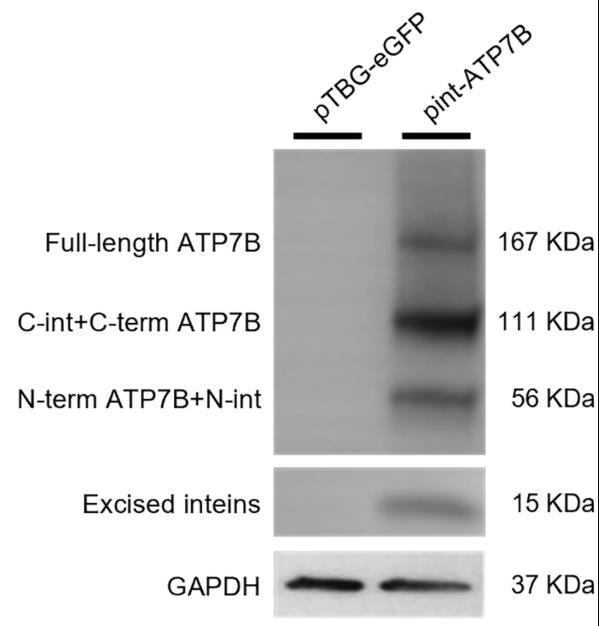
Fig3. Western blot analysis on extracts from ATP7B-KO HepG2 cells co-transfected with int-ATP7B constructs.
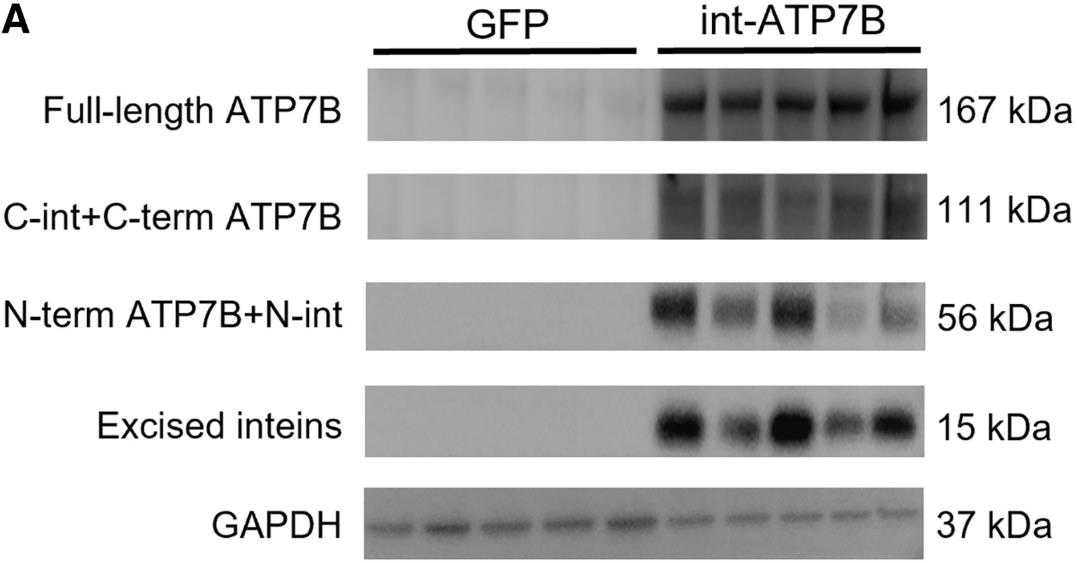
Fig4. Western blot analysis using anti-FLAG antibody of whole liver lysate from Atp7b−/− mice.
Quality Guarantee
High Purity
.jpg)
Fig1. SDS-PAGE (ATP7B-1014H)
.
.jpg)
Fig2. SDS-PAGE (ATP7B-468H)
Involved Pathway
ATP7B involved in several pathways and played different roles in them. We selected most pathways ATP7B participated on our site, such as Ion channel transport,Ion transport by P-type ATPases,Transmembrane transport of small molecules, which may be useful for your reference. Also, other proteins which involved in the same pathway with ATP7B were listed below. Creative BioMart supplied nearly all the proteins listed, you can search them on our site.
| Pathway Name | Pathway Related Protein |
|---|---|
| Ion transport by P-type ATPases | ATP1A1A.5,FXYD6-FXYD2,ATP1A1A.4,ATP13A5,ATP2B1B,CUTC,CCC2,ATP1A1A.2,ATP1A1A.3,ATP2B3A |
| Ion channel transport | TSC22D3,ATP1A1A.4,ATP13A1,GABRR2A,Ano3,ANO10,BEST3,MCOLN2,CLIC2,ATP10A |
| Transmembrane transport of small molecules | SLC43A1B,SLC17A3,CLCN7,TRPV3,SLC43A2B,SLC5A2,RHCGL1,SLC22A16,SLC7A3A,SLC24A5 |
Protein Function
ATP7B has several biochemical functions, for example, ATP binding,copper ion binding,copper-exporting ATPase activity. Some of the functions are cooperated with other proteins, some of the functions could acted by ATP7B itself. We selected most functions ATP7B had, and list some proteins which have the same functions with ATP7B. You can find most of the proteins on our site.
| Function | Related Protein |
|---|---|
| copper ion binding | IL1A,AOC2,HIST2H3A,ABP1,SCO2,LOXL3,CP,S100V2,ANG,MOXD2 |
| ATP binding | CAMKK1B,TXK,IDH3G,UBE2C,ATAD2B,PYGL,TWF2,ATP1A1B,NME6,SPATA5 |
| copper-exporting ATPase activity | ATP7A |
| protein binding | CCDC53,ARNTL1A,GALNT2,SLC19A2,GBP5,FKBP5,SEPT11,SPTAN1,CENPA,ARL6IP5 |
Interacting Protein
ATP7B has direct interactions with proteins and molecules. Those interactions were detected by several methods such as yeast two hybrid, co-IP, pull-down and so on. We selected proteins and molecules interacted with ATP7B here. Most of them are supplied by our site. Hope this information will be useful for your research of ATP7B.
Resources
Related Services
Related Products
References
- Palm-Espling, ME; Lundin, C; et al. Interaction between the Anticancer Drug Cisplatin and the Copper Chaperone Atox1 in Human Melanoma Cells. PROTEIN AND PEPTIDE LETTERS 21:63-68(2014).
- Valtorta, S; Belloli, S; et al. Comparison of F-18-Fluoroazomycin-Arabinofuranoside and Cu-64-Diacetyl-Bis(N4-Methylthiosemicarbazone) in Preclinical Models of Cancer. JOURNAL OF NUCLEAR MEDICINE 54:1106-1112(2013).


