PRTN3
-
Official Full Name
proteinase 3 -
Overview
PRTN3 is a serine protease enzyme expressed mainly in neutrophil granulocytes. Its exact role in the function of the neutrophil is unknown but in human neutrophils proteinase 3 contributes to the proteolytic generation of antimicrobial peptides. It is also the epitope of anti-neutrophil cytoplasmic antibodies (ANCAs) of the cANCA (cytoplasmic subtype) class, a type of antibody frequently found in the disease Wegener's granulomatosis. -
Synonyms
MBN;MBT;NP4;P29;PR3;ACPA;AGP7;PR-3;CANCA;C-ANCA;myeloblastin;C-ANCA antigen;wegener autoantigen;leukocyte proteinase 3;neutrophil proteinase 4;azurophil granule protein 7;serine proteinase, neutrophil;Wegener granulomatosis autoantigen;proteinase 3;NP-4;EC 3.4.21.76;EC 3.4.21
Recombinant Proteins
- Human
- Mouse
- Rat
- E.coli
- HEK293
- Mammalian Cell
- Wheat Germ
- Mouse
- Peripheral blood leukocytes
- Mammalian cells
- Human Azurophil Granules
- Neutrophils
- In Vitro Cell Free System
- His
- Non
- Flag
- His&Fc&Avi
- Myc&DDK
- GST
- His&Myc
Background
What is PRTN3 protein?
PRTN3 gene (proteinase 3) is a protein coding gene which situated on the short arm of chromosome 19 at locus 19p13. The protein encoded by this gene is involved in enzyme binding activity, serine endopeptidase activity, and signal receptor binding activity. Its expression is limited in bone marrow and has specific expression patterns in a variety of tissues. It is mainly found in the osmophilic granules of neutrophils and is involved in a variety of biological processes, including differentiation of mature conventional dendritic cells, exoteric proteolysis of membrane proteins, and exudation of neutrophils. The PRTN3 protein is consisted of 256 amino acids and PRTN3 molecular weight is approximately 27.8 kDa.
What is the function of PRTN3 protein?
PRTN3 also known as PR3, is a neutrophil-specific serine protease, whose biological functions are mainly involved in inflammatory response and immune regulation. PRTN3 is highly expressed in the osmophilic granules of neutrophils and is able to break down a variety of proteins, including cell wall components of bacteria and fungi, thus playing a role in innate immune defense. In addition, PRTN3 is involved in the processing and presentation of antigens, affecting the maturation and function of dendritic cells, and thus regulating the adaptive immune response.
PRTN3 related signaling pathway
Protease 3 related signaling pathways mainly include protease activating receptor (PARs) signaling pathway and neutrophil extracellular trap (NETs) formation pathway. Protease activating receptors (PARs) are a class of seven transmembrane G-protein-coupled receptors, including four subtypes, which can be cleaved and activated by a variety of proteases, and then participate in the regulation of biological processes such as inflammation, coagulation, and immune response. In the NETs formation pathway, protease 3, as a serine protease in neutrophils, is involved in the formation of NETs, which play a role in trapping and killing microorganisms, but may also lead to inflammatory damage and autoimmune disease.
PRTN3 related diseases
The PRTN3 protein has been implicated in a variety of diseases, particularly those related to the immune system. It is highly expressed in neutrophils and is involved in inflammatory response and immune regulation. PRTN3 is strongly associated with ANCA-associated vasculitis (AAV), in which PR3-ANCA (an autoantibody against PRTN3) is associated with AAV subtypes such as Wegener's granulomatosis. In addition, PRTN3 plays an important role in chronic inflammatory diseases such as chronic obstructive pulmonary disease (COPD) and pulmonary cystic fibrosis. In acute myeloid leukemia (AML), the loss of PRTN3 has been found to promote the differentiation of myeloid cells, potentially countering the development of leukemia.

Fig1. Summary of the actions of Proteinase 3 (PR3), which likely impact on COPD and other systemic diseases. (Helena Crisford, 2018)
Bioapplications of PRTN3
Recombinant human protease 3 (rhPRTN3) has many potential applications in biomedical research. As a key serine protease in neutrophils, rhPRTN3 plays an important role in the study of immune response, inflammatory processes, and the pathogenesis of ANCA-associated vasculitis. rhPRTN3 has also shown its value in cancer research, especially in exploring its function in the tumor microenvironment and as a potential biomarker. Studies of the activity and function of rhPRTN3 have contributed to the development of new therapeutic strategies, including the design of targeted inhibitors and immunotherapy drugs. Recombinant expression of active PR3 overcomes the need for enzyme supplies from human blood and in addition allows studies on the influence of mutations on enzyme activity and ligand binding.
Case Study
Case Study 1: Huan Liu, 2024
This study reveals that deleting PRTN3 in mice triggers myeloid differentiation, suggesting it acts as a negative regulator in this process. PRTN3 interacts with STAT3, promoting its degradation and reducing its activation, which is crucial for myeloid differentiation. Inhibiting STAT3 partially reverses the effects of PRTN3 deletion. Furthermore, PRTN3 deficiency in acute myeloid leukemia (AML) cells enhances their differentiation into functional neutrophils, improving survival rates, indicating PRTN3's potential as a therapeutic target for leukemia.
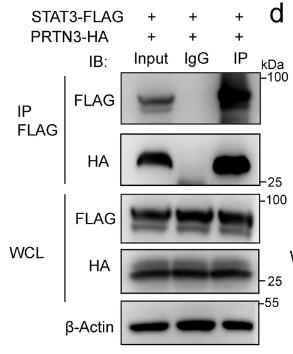
Fig1. WB analysis of Co-IP complex confirmed that STAT3-Flag interacted with PRTN3-HA in HEK 293 T cells.

Fig2. SDS/PAGE analysis of hPR3-generated fragments of STAT3.
Case Study 2: Fahimeh Khorsand, 2024
Recombinant neutrophil proteinase 3 (rPR3), produced using a baculovirus expression system, serves as a valuable tool for drug discovery in inflammatory lung diseases. This method provides a consistent source of active PR3 for inhibitor screening, enzymatic assays, and structural analysis, eliminating the need for human blood-derived enzyme. rPR3 in this study showed similar activity to natural human PR3 in fluorescence-based assays and was used to develop a surface plasmon resonance assay for studying PR3-ligand interactions, making it an effective alternative for in vitro enzymatic research.

Fig3. SDS-PAGE showing the purity of rPR3.

Fig4. Sensorgrams of α1-antitrypsin binding to rPR3.
Quality Guarantee
High Purity
.jpg)
Fig1. SDS-PAGE (PRTN3-1996H)
.
.jpg)
Fig2. SDS-PAGE (PRTN3-6019H)
Involved Pathway
PRTN3 involved in several pathways and played different roles in them. We selected most pathways PRTN3 participated on our site, such as C-MYB transcription factor network,Common Pathway of Fibrin Clot Formation,Formation of Fibrin Clot (Clotting Cascade), which may be useful for your reference. Also, other proteins which involved in the same pathway with PRTN3 were listed below. Creative BioMart supplied nearly all the proteins listed, you can search them on our site.
| Pathway Name | Pathway Related Protein |
|---|---|
| Hemostasis | PDE10A,SLC16A8,MAFF,ZFPM1,KIF15,DOCK6,AKAP1B,ANGPT2A,CFD,IGF2B |
| C-MYB transcription factor network | COL1A2,ELANE,CDKN1B,CDK6,CSF1R,CREBBP,CEBPB,TOM1,MYB,PTCRA |
| Common Pathway of Fibrin Clot Formation | F13A1B,CFHL3,CD177,PROCR,F13A1A.1,LOC100514666,SERPINA1L,CFHL2,SERPINE2,PROCA |
| Formation of Fibrin Clot (Clotting Cascade) | CFHL2,F9A,F13A1B,CFHL4,SERPINA1L,F3B,PROCA,F13A1A.1,CFHL1,F9B |
Protein Function
PRTN3 has several biochemical functions, for example, enzyme binding,serine-type endopeptidase activity,serine-type peptidase activity. Some of the functions are cooperated with other proteins, some of the functions could acted by PRTN3 itself. We selected most functions PRTN3 had, and list some proteins which have the same functions with PRTN3. You can find most of the proteins on our site.
| Function | Related Protein |
|---|---|
| serine-type peptidase activity | PRSS52,IMMP2L,TMPRSS11E,HTRA1B,GZMK,F9B,HPN,CMA1,KLK1B26,FAP |
| serine-type endopeptidase activity | CTSG,LPA,ST14A,TMPRSS11B,AZU1,GZME,KLK2,Prss32,PROZ,PRSS37 |
| enzyme binding | PLSCR4,YWHAB,TSPAN5,HDAC1,CREB1,TNKS2,ZNF346,KMT2E,CYP2A6,RAD9A |
Interacting Protein
PRTN3 has direct interactions with proteins and molecules. Those interactions were detected by several methods such as yeast two hybrid, co-IP, pull-down and so on. We selected proteins and molecules interacted with PRTN3 here. Most of them are supplied by our site. Hope this information will be useful for your research of PRTN3.
SMAD3;A2M;P/V/C
Resources
Related Services
Related Products
References
- Alberici, F; Martorana, D; et al. Genetics of ANCA-associated vasculitides: HLA and beyond. CLINICAL AND EXPERIMENTAL RHEUMATOLOGY 32:S90-S97(2014).
- Lyons, PA; Rayner, TF; et al. Genetically Distinct Subsets within ANCA-Associated Vasculitis. NEW ENGLAND JOURNAL OF MEDICINE 367:214-223(2012).


