PLK4
-
Official Full Name
polo-like kinase 4 -
Overview
This gene encodes a member of the polo family of serine/threonine protein kinases. The protein localizes to centrioles, complex microtubule-based structures found in centrosomes, and regulates centriole duplication during the cell cycle. Three alternatively spliced transcript variants that encode different protein isoforms have been found for this gene. [provided by RefSeq, Jun 2010] -
Synonyms
PLK4;polo-like kinase 4;SAK;STK18;serine/threonine-protein kinase PLK4;PLK-4;Snk akin kinase;serine/threonine kinase 18;serine/threonine-protein kinase 18;serine/threonine protein kinase SAK;serine/threonine-protein kinase Sak
Recombinant Proteins
- Human
- Mouse
- Zebrafish
- E.coli
- Mammalian Cell
- Sf9 Insect Cell
- HEK293
- Insect Cell
- E. coli
- GST
- His
- Non
- N-Twin strep and C-His&Flag
- His&Fc&Avi
Background
What is PLK4 Protein?
PLK4 is a type of kinase that helps control how cells duplicate their centrioles during the cell cycle. It keeps this process in check by adding phosphate groups and interacting with other proteins. Normally, there's not a lot of PLK4 around, and its levels are tightly regulated. If there's too much PLK4, cells end up with extra centrioles; too little, and they can't make centrioles properly. Both issues can cause problems with cell division, possibly leading to cancer. PLK4 is crucial for cell growth, as shown in studies with mice. Lower PLK4 levels can reduce cell movement, hinting that PLK4 might be linked to cancer spread. Overexpression of PLK4 is seen in several cancers and might also contribute to resistance against chemotherapy, making PLK4 a potential target for cancer treatments.What is the Function of PLK4 Protein?
PLK4 is a protein that plays a big role in helping cells divide properly. It ensures each centrosome duplicates just once before mitosis by interacting with other key proteins. Besides its main job in centrosome replication, PLK4 is also crucial for keeping cellular structures intact and helping in cilia formation, both important during cell division. Normally, PLK4 levels are carefully controlled. If there's too much, cells might form extra centrioles; too little, and they struggle to make enough. This imbalance can lead to issues like chromosome missegregation, hindering cell cycle progress and potentially causing cancer. This protein is vital for cell growth, as shown in studies with genetically modified mice. Interestingly, lower PLK4 levels reduce cell movement, while higher levels are linked to cancer spread in various types, like breast, lung, and liver cancer. High PLK4 levels also connect to resistance against chemotherapy, making it a potential target for cancer treatment strategies.PLK4 Related Signaling Pathway
PLK4 protein is key in various signaling pathways, especially in cell cycle control, cell growth, and cancer development. It works by binding to AKT1 and phosphorylating specific sites, which kicks off the PI3K/AKT/mTOR pathway, fueling tumor growth and glucose metabolism in gliomas. PLK4 boosts the phosphorylation of AKT1, affecting glucose metabolism even more. During epithelial-mesenchymal transition (EMT), PLK4 helps drive changes by downregulating E-cadherin and upregulating EMT factors through the PI3K/Akt pathway. In colorectal cancer, PLK4 promotes similar transitions via the Wnt/β-catenin pathway. It's also linked to the NF-kB pathway, crucial for gene expression needed for centrosome duplication, with PLK4 being a direct target. Moreover, p53 can downregulate PLK4, opening another avenue for cancer cell interactions that promote cancer progression. Hence, PLK4 plays a significant role in managing the cell cycle, fostering cancer growth, and impacting immune infiltration in tumors.
Fig1. Schematic representation of PLK4 signaling in cancer with details of selected PLK4 inhibitors. (Debra R Garvey, 2021)
PLK4 Related Diseases
PLK4 is linked to various diseases, especially cancer. It's key in centrosome replication, and its abnormal expression is tied to cancers like liver, colorectal, pancreatic, and prostate. In acute myeloid leukemia (AML), particularly with TP53 mutations, PLK4 emerges as a promising target. Research shows PLK4 acts in TP53-mutant AML, which often resists chemo but responds well to prolonged PLK4 inhibition. Blocking PLK4 can cause DNA damage, aging cells, and cell division issues, and it's connected to the cGAS-STING pathway and immune activation. In neuroblastoma, PLK4 levels correlate with clinical features and survival rates; higher PLK4 means worse outcomes and helps cancer progress via the PI3K/Akt pathway. This highlights PLK4's role in cancer and its potential as a treatment target.Bioapplications of PLK4
PLK4 protein holds promising potential in biomedical research and cancer treatment. As a key regulator in centrosome replication, its abnormal expression is tied to various cancers. Research on PLK4's structure and function helps unravel the molecular mechanisms behind centrosome duplication and cell cycle control. PLK4's activity sparks centrosome formation through interactions with proteins like STIL and SAS6. It's also pivotal in tumor growth and resistance, particularly by activating the AKT1 pathway to fuel glioma progression. In terms of drug development, there's a strong focus on PLK4 inhibitors, which can halt cancer cell growth and induce cell cycle arrest. Recent advances explore inhibitor activity, specificity, drug-like properties, and interactions with PLK4 protein structures, as well as combination therapies. These efforts pave the way for new cancer treatments and drug discoveries.Case Study
Case Study 1: Grossmann J. et al. Life Sci Alliance. 2024
Centrioles are key for building centrosomes and cilia, and they duplicate once per cell cycle with the help of polo-like kinase 4 (PLK4). To avoid excessive centriole duplication—a cancer trait—PLK4 levels must be controlled. We've found that the E3 ligase complex, Cullin4A/B-DDB1-DCAF1 (CRL4DCAF1), targets PLK4 for breakdown in human cells. DCAF1 binds and tags PLK4 for degradation during the G2 phase, stopping early centriole duplication in mitosis. Unlike regulation by SCFβ-TrCP, PLK4's interaction with DCAF1 doesn't rely on its kinase activity but involves its polo-boxes 1 and 2. This suggests DCAF1 promotes PLK4 degradation without β-TrCP. Therefore, the SCFSlimb/β-TrCP and CRL4DCAF1 pathways offer complementary methods to regulate PLK4 levels and prevent excess centriole duplication.-
 Fig1. Flag-PLK4 and HA-DCAF1 were overexpressed in HEK293T cells for 48 h.
Fig1. Flag-PLK4 and HA-DCAF1 were overexpressed in HEK293T cells for 48 h. -
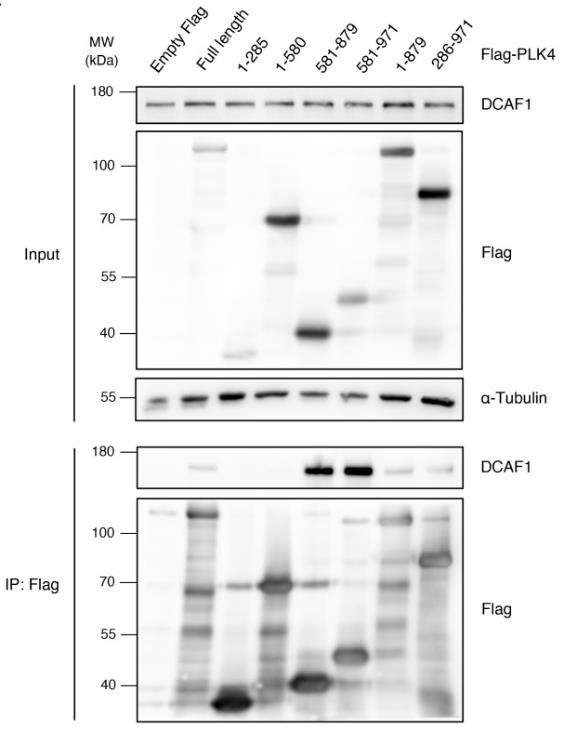 Fig2. Flag-PLK4 full-length or different truncated fragments were overexpressed in HEK293T cells for 48 h.
Fig2. Flag-PLK4 full-length or different truncated fragments were overexpressed in HEK293T cells for 48 h.
Case Study 2: Scott P. et al. J Cell Biol. 2023
Polo-like kinase 4 (PLK4) is crucial for making centrioles, but it's not clear how it picks one site for new centriole growth. Using advanced microscopy, we've found that PLK4 sits at specific spots on the parent centriole. Although it can be found at multiple places, PLK4 usually clusters at a main site to guide new centriole formation. When PLK4 activity is blocked, it sticks to the centriole more and spreads to up to nine spots. Self-phosphorylation of a particular linker helps release active PLK4, allowing it to choose just one spot for growth. Stopping this phosphorylation keeps PLK4 stuck, resulting in too many growth sites and excess centrioles. So, self-phosphorylation is key for PLK4 positioning and selecting just one site for centriole duplication.-
 Fig3. Graph showing the amount of PLK4 localized at individual sites on parent centrioles.
Fig3. Graph showing the amount of PLK4 localized at individual sites on parent centrioles. -
 Fig4. Graph showing the amount of PLK4 localized at individual sites of early G1 RPE1 or DLD1 cells with an α-tubulin cytokinetic bridge.
Fig4. Graph showing the amount of PLK4 localized at individual sites of early G1 RPE1 or DLD1 cells with an α-tubulin cytokinetic bridge.
Quality Guarantee
High Purity
-
.jpg) Fig1. SDS-PAGE (PLK4-01H)
Fig1. SDS-PAGE (PLK4-01H)
High Bioactivity
-
.jpg) Fig2. Activity Data (PLK4-39H)
Fig2. Activity Data (PLK4-39H)
Involved Pathway
PLK4 involved in several pathways and played different roles in them. We selected most pathways PLK4 participated on our site, such as Anchoring of the basal body to the plasma membrane,Assembly of the primary cilium,Cell Cycle, which may be useful for your reference. Also, other proteins which involved in the same pathway with PLK4 were listed below. Creative BioMart supplied nearly all the proteins listed, you can search them on our site.
| Pathway Name | Pathway Related Protein |
|---|---|
| Anchoring of the basal body to the plasma membrane | DCTN2,TTBK2,ACTR1A,NPHP4,MAPRE1,SCLT1,TUBB4A,DCTN1A,CCP110,SDCCAG8 |
| G2/M Transition | MAPRE1B,TUBG2,TCTN2,FOXM1,DCTN2,CLASP1A,SDCCAG8,ODF2,MAPRE1,CEP135 |
| Loss of Nlp from mitotic centrosomes | NDE1,CEP78,MAPRE1B,PCM1,CKAP5,CP110,DCTN1A,MAPRE1A,NEDD1,CEP57 |
| Centrosome maturation | SSNA1,DCTN3,CLASP1A,CCP110,CENPJ,MAPRE1B,TUBB4B,CDK11B,DYNC1H1,PCM1 |
| FoxO signaling pathway | PRMT1,PIK3CB,PIK3R3B,INSB,MAPK14B,CDKN1BA,AKT3A,FOXG1,IGF1R,CCNB3 |
| Assembly of the primary cilium | IFT-20,EXOC3,BBS13,UNC119B,SDCCAG8,KIFAP3,TTC30B,MARK4,ARL3,RAB3IP |
| Cell Cycle, Mitotic | GINS1,CENPH,ARPP19,GINS3,MAU2,PMF1,KIF2B,ARPP19B,NCAPD3,TUBG1 |
| Cell cycle | CCNA1,BLZF1,CCP110,GINS1,AKAP9,MDM2,SMC1A,DCTN1A,NIPBLA,NCAPH |
-
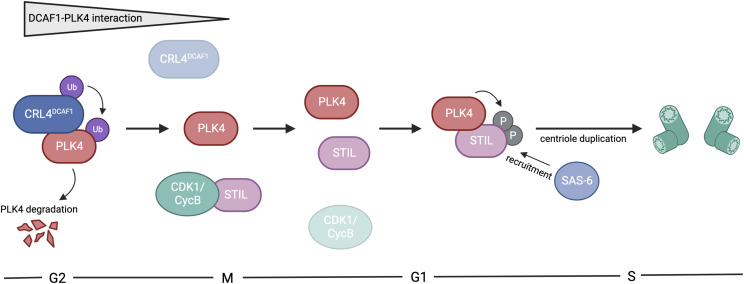 Fig1. CRL4DCAF1 regulates polo-like kinase 4 (PLK4) in centriole duplication. (Josina Grossmann, 2024)
Fig1. CRL4DCAF1 regulates polo-like kinase 4 (PLK4) in centriole duplication. (Josina Grossmann, 2024) -
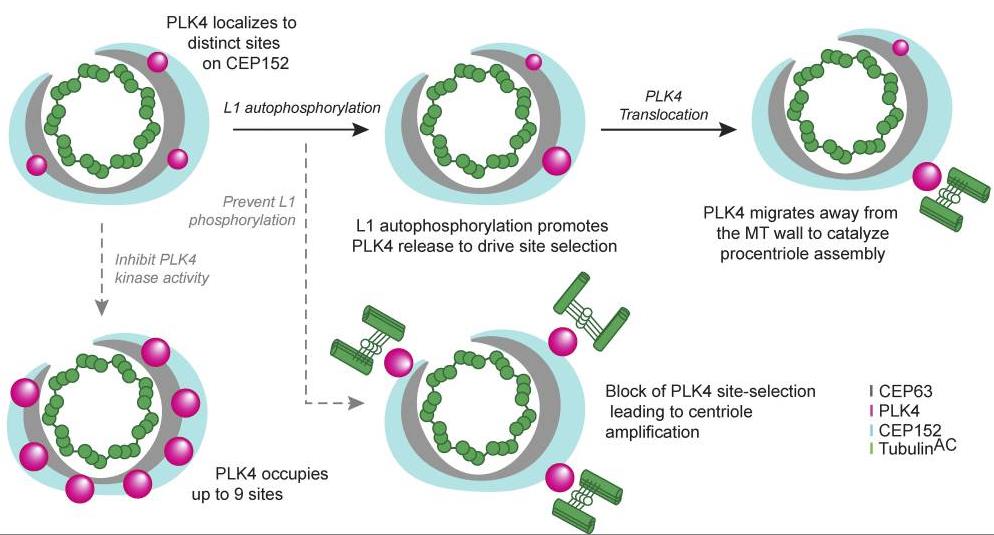 Fig2. Model for PLK4 site selection. (Phillip Scott, 2023)
Fig2. Model for PLK4 site selection. (Phillip Scott, 2023)
Protein Function
PLK4 has several biochemical functions, for example, ATP binding,identical protein binding,protein binding. Some of the functions are cooperated with other proteins, some of the functions could acted by PLK4 itself. We selected most functions PLK4 had, and list some proteins which have the same functions with PLK4. You can find most of the proteins on our site.
| Function | Related Protein |
|---|---|
| protein binding | TRIM50,NBR1,DNALI1,LIN28A,PI4KB,BFSP1,MIPOL1,CARHSP1,RTP3,TAF9B |
| ATP binding | DHX57,TRIP13,GSG2,MAP4K4,RPS6KC1,STK33,UBE2O,PTK7A,CLK2A,PFKM |
| protein serine/threonine kinase activity | RPS6KA4,TGFBR1B,ACVR1B,MTOR,MAP4K6,DYRK2,ICK,NEK11,BSK146,PRKACBB |
| identical protein binding | G6PDX,POU3F2,MSANTD3,GNPNAT1,CLDN11,FKBP8,PUF60,TOPBP1,DAPK1,RELA |
Interacting Protein
PLK4 has direct interactions with proteins and molecules. Those interactions were detected by several methods such as yeast two hybrid, co-IP, pull-down and so on. We selected proteins and molecules interacted with PLK4 here. Most of them are supplied by our site. Hope this information will be useful for your research of PLK4.
ARR3;TCEB3;C1orf109;SFN;CENPJ;xmd8-92;TAX1BP3;CHD3;MTUS2;FAM46C;czc8004
Resources
Related Services
Related Products
References


