PARG
-
Official Full Name
poly (ADP-ribose) glycohydrolase -
Overview
Poly(ADP-ribose) glycohydrolase (PARG) is the major enzyme responsible for the catabolism of poly(ADP-ribose), a reversible covalent-modifier of chromosomal proteins. The protein is found in many tissues and may be subject to proteolysis generating smaller, active products. [provided by RefSeq, Jul 2008] -
Synonyms
PARG;poly (ADP-ribose) glycohydrolase;PARG99;poly(ADP-ribose) glycohydrolase;mitochondrial poly(ADP-ribose) glycohydrolase;poly(ADP-ribose) glycohydrolase 60 kDa isoform
Recombinant Proteins
- Human
- Rat
- Insect Cells
- E.coli
- Wheat Germ
- HEK293
- Mammalian Cells
- In Vitro Cell Free System
- His
- GST
- Non
- Avi
- Fc
| Cat.# | Product name | Source (Host) | Species | Tag | Protein Length | Price |
|---|---|---|---|---|---|---|
| PARG-31H |
Active Recombinant Human PARG protein, His-tagged

|
Insect Cells | Human | His | ||
| PARG-1528H | Recombinant Human PARG, GST-tagged | E.coli | Human | GST | 617-976aa | |
| PARG-1529H | Recombinant Human PARG protein, GST-tagged | Wheat Germ | Human | GST | 1 a.a. - 976 a.a. | |
| PARG-1530H | Recombinant Human PARG Protein, His-tagged | E.coli | Human | His | 1-976 a.a. | |
| PARG-33H | Recombinant Human PARG Protein, His-tagged | HEK293 | Human | His | Ser448~Thr976 | |
| PARG-125H | Recombinant Human PARG protein, GST-tagged | Wheat Germ | Human | GST | 357-467 a.a. |
|
| PARG-4272R | Recombinant Rat PARG Protein | Mammalian Cells | Rat | His |
|
|
| PARG-3434HCL | Recombinant Human PARG 293 Cell Lysate | HEK293 | Human | Non |
|
|
| PARG-1171H | Recombinant Human PARG Protein, His-tagged | HEK293 | Human | His | Ser448-Thr976 |
|
| PARG-2548H | Recombinant Human PARG Protein, His-tagged | E.coli | Human | His | Ser448-Thr976 |
|
| PARG-3934R | Recombinant Rat PARG Protein, His (Fc)-Avi-tagged | HEK293 | Rat | Avi&Fc&His |
|
|
| PARG-3934R-B | Recombinant Rat PARG Protein Pre-coupled Magnetic Beads | HEK293 | Rat |
|
||
| PARG-566HF | Recombinant Full Length Human PARG Protein, GST-tagged | In Vitro Cell Free System | Human | GST | Full L. 976 amino acids |
|
| PARG-87H | Recombinant Human PARG protein, GST-tagged | E.coli | Human | GST | 183-441 aa |
|
Background
What is PARG Protein?
PARG, or poly(ADP-ribose) glycohydrolase, is the main enzyme responsible for breaking down poly(ADP-ribose) (PAR), a reversible chromosomal protein modifier involved in DNA damage response. PARG is expressed in various tissues and may be cleaved into smaller active forms. By hydrolyzing the glycosidic bonds in PAR chains, PARG releases ADP-ribose and oligo(ADP-ribose), which might signal genotoxic stress. Its role isn't limited to DNA repair; it also affects DNA replication, transcription, and chromatin structure. PARG activity is crucial for genome integrity and cell survival. There are five PARG isoforms from alternative splicing, functioning in both the nucleus and cytoplasm, helping prevent mitotic disasters from radiation and maintaining replication fork stability via BRCA2 pathways. PARG knockout cells become more sensitive to chemotherapy and radiation and may face more specific killing in BRCA2-deficient cancer cells. Additionally, PARG could help prevent cytoplasmic PAR buildup and certain PAR-mediated cell death types. Thus, PARG plays a key role in DNA repair, genome maintenance, and potential cancer treatments.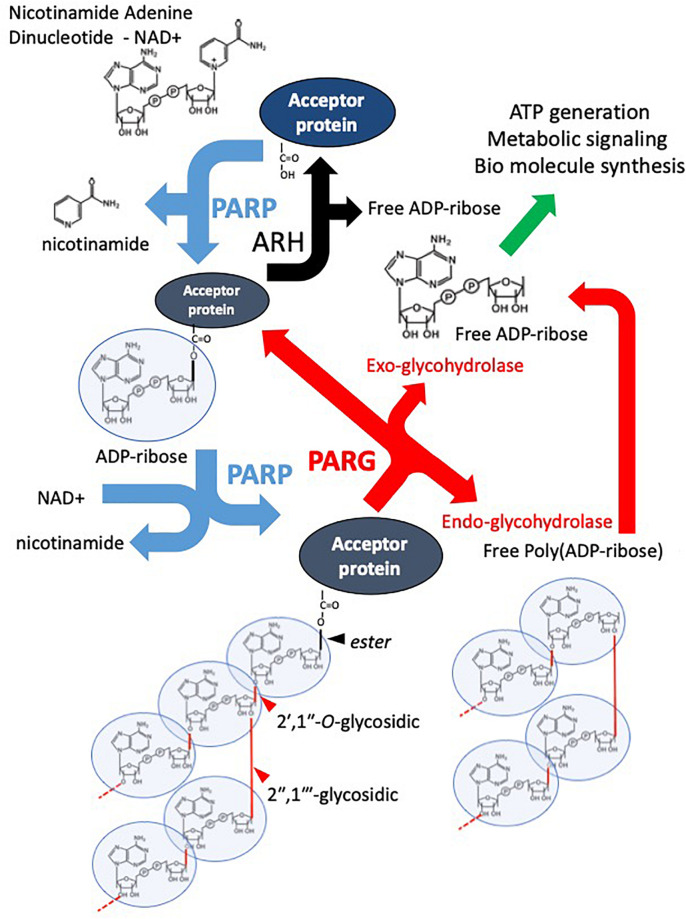
Fig1. Overview of the PAR Cycle. (Daniel Harrision, 2020)
What is the Function of PARG Protein?
PARG, or poly(ADP-ribose) glycohydrolase, is a key player in cells, involved in DNA repair, replication, chromatin remodeling, transcription regulation, mitochondrial function, and apoptosis. It primarily breaks down PAR chains, created by PARP enzymes, into ADP-ribose through its glycosidase activities. After DNA damage, PAR metabolism is quickly handled by PARP-1, PARP-2, and PARG, with PAR lasting only about a minute. Accumulated PAR is harmful and linked to caspase-independent cell death. PARG is vital for genome integrity, as its absence causes harmful PAR buildup. PARG is also linked to cancer, where abnormal PARylation aids tumor growth and invasion. Targeting PARG pharmacologically shows promise in cancer therapy. Thus, PARG is crucial in DNA repair, genome maintenance, and cancer treatment.PARG Related Signaling Pathway
PARG, or poly(ADP-ribose) glycohydrolase, is vital in DNA damage response and genomic maintenance. It partners with PARP proteins to regulate PAR levels for effective DNA repair. In SSBR, PARG increases SSBs and impacts repair rates. Its absence makes cells more susceptible to DNA damage. PARG breaks PAR chains to release ADP-ribose, signaling stress. It prevents cytoplasmic PAR accumulation, aids replication fork stability, and DSB repair, particularly through BRCA2. PARG's role at DNA damage sites, interacting with PCNA and PARP1, averts radiation-induced cell issues. Knockout of PARG increases sensitivity to treatments, notably in BRCA2-deficient cells. Therefore, PARG is key for DNA repair, genome integrity, and cancer therapy, supporting cell survival.PARG Related Diseases
PARG protein is tied to various diseases, notably playing a key role in DNA damage repair, genomic stability, and cancer treatment. It regulates PAR levels by breaking down glycosidic bonds in PAR chains, crucial for maintaining genomic integrity and cell survival. Beyond just fixing DNA, it's also about DNA replication, chromatin structure, and transcription regulation. In cancer therapy, PARG inhibitors hold big promise, especially for tumors with faulty HR DNA repair paths, like those with BRCA1/2 mutations. Knocking down or inhibiting PARG can make cells more sensitive to DNA-damaging agents, leading to synthetic lethality, and it's vital in preventing cytoplasmic PAR buildup and parthanatos (a type of caspase-independent cell death mediated by PAR). Plus, PARG plays a hand in regulating transcription and gene expression. Thus, PARG protein is a significant player in various diseases' emergence and progression, especially in cancer treatment, where PARG inhibitors as a budding strategy offer a key opportunity for precision medicine.Bioapplications of PARG
PARG protein (Poly(ADP-ribose) glycohydrolase) is a big deal in biomedical research and clinical treatment, especially when it comes to cancer. Developing PARG inhibitors is a hot topic right now because they can exploit and worsen cancer cells' replication flaws, potentially complementing PARP inhibitors when tackling cancers with various genomic instabilities. These inhibitors stop PARG from breaking down PAR chains, leading to cancer cell death and working similarly to the PARP inhibitor Olaparib. They're set to be assessed across different cancer therapies due to their unique presence—there's no mammalian equivalent to PARG, meaning potentially fewer off-target effects compared to the 17 other enzymes in the human PARP family. Also, PARG inhibitors show promise in overcoming cancer cells' resistance to PARP inhibitors, and they might be combined with them for better results. So, PARG protein and its inhibitors have a bright future in cancer treatment, offering new paths for anti-cancer strategies.Case Study
Case Study 1: Yan J. et al. Biochem Genet. 2024
Esophagus cancer (EC) is aggressive and spreads easily. Poly(ADP-ribose) glycohydrolase (PARG) is key in DNA repair and replication, and this study explored its impact on EC. Using various tests, the study found that PARG is highly expressed in EC tissues and cells. Reducing PARG levels decreased cell growth, invasion, migration, adhesion, and epithelial-mesenchymal transition. On the flip side, more PARG boosted these activities. PARG also activated the Wnt/β-catenin pathway but not the STAT or Notch pathways. The Wnt/β-catenin inhibitor XAV939 partly blocked PARG's effects. Thus, PARG enhances EC's malignancy by activating the Wnt/β-catenin pathway.-
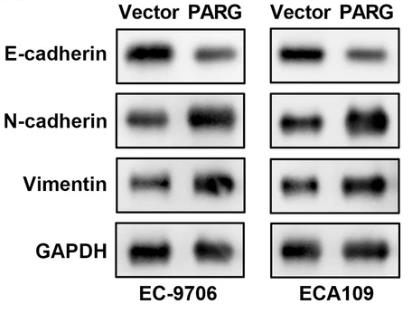 Fig1. Western blot examined the levels of EMT-related factors.
Fig1. Western blot examined the levels of EMT-related factors. -
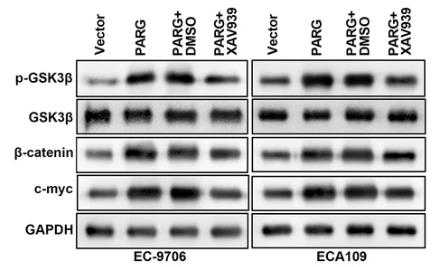 Fig2. Inactivation of the Wnt/β-catenin pathway abrogates cell metastasis facilitated by PARG.
Fig2. Inactivation of the Wnt/β-catenin pathway abrogates cell metastasis facilitated by PARG.
Case Study 2: Ray Chaudhuri A. et al. Mol Cell Biol. 2015
Poly(ADP-ribosyl)ation (PAR) plays a crucial role in how cells respond to DNA damage and maintain genome stability. Despite having 17 PARP genes, only one PARG is responsible for PAR breakdown. This study examined PARG's role in human chromosome replication. It found that reducing PARG disrupts cell growth and DNA synthesis, causing H2AX phosphorylation linked to replication. PARG shortage or inhibition similarly slows replication forks as mild chemotherapy does. Electron microscopy showed an increase in reversed forks and ssDNA gaps in PARG-deficient cells, without visible chromosomal breaks. However, these cells activated ATM and ATR pathways and recruited DSB repair factors like 53BP1 and RAD51 to chromatin. The findings indicate that PAR breakdown is vital for restarting replication after damage and preventing unnecessary repair factor recruitment. This suggests a potential for targeting PARG in cancer chemotherapy.-
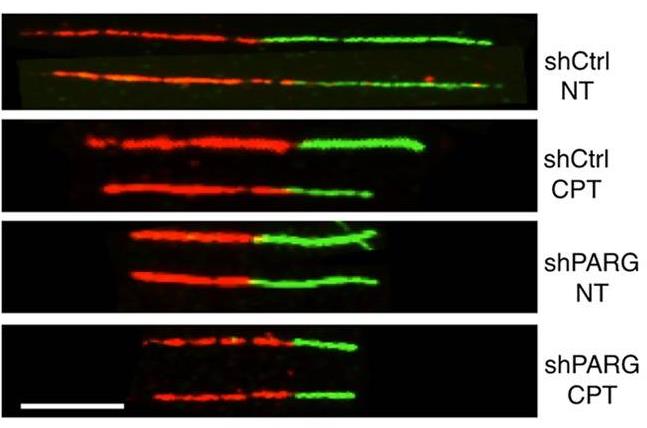 Fig3. Schematic experimental conditions for DNA replication track analysis.
Fig3. Schematic experimental conditions for DNA replication track analysis. -
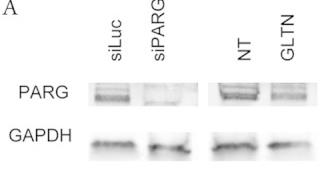 Fig4. PARG levels after siRNA-mediated depletion were detected by immunoblotting.
Fig4. PARG levels after siRNA-mediated depletion were detected by immunoblotting.
Quality Guarantee
High Purity
-
.jpg) Fig1. SDS-PAGE (PARG-2548H)
Fig1. SDS-PAGE (PARG-2548H) -
.jpg) Fig2. SDS-PAGE (PARG-1171H)
Fig2. SDS-PAGE (PARG-1171H)
Involved Pathway
PARG involved in several pathways and played different roles in them. We selected most pathways PARG participated on our site, such as , which may be useful for your reference. Also, other proteins which involved in the same pathway with PARG were listed below. Creative BioMart supplied nearly all the proteins listed, you can search them on our site.
| Pathway Name | Pathway Related Protein |
|---|
-
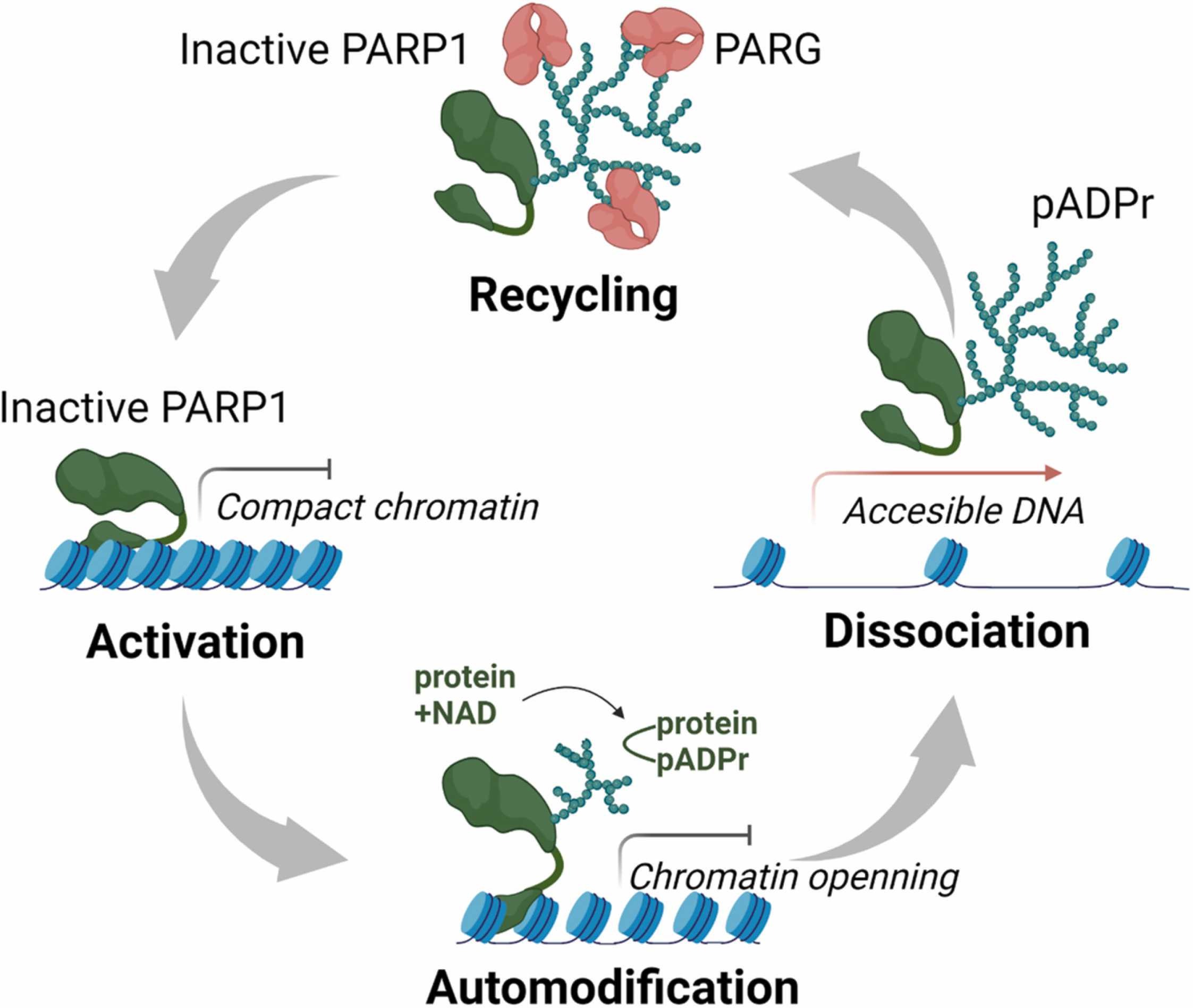 Fig1. PARG regulates poly(ADP-ribose) turnover in the cell. (Yaroslava Karpova, 2022)
Fig1. PARG regulates poly(ADP-ribose) turnover in the cell. (Yaroslava Karpova, 2022) -
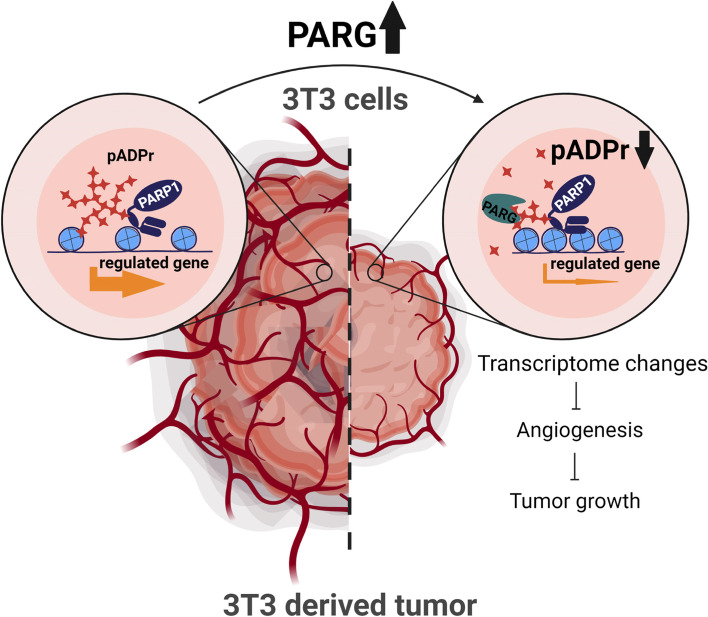 Fig2. The proposed role of poly(ADP-ribosyl) ation pathway in tumorigenesis. (Sarah Johnson, 2022)
Fig2. The proposed role of poly(ADP-ribosyl) ation pathway in tumorigenesis. (Sarah Johnson, 2022)
Protein Function
PARG has several biochemical functions, for example, . Some of the functions are cooperated with other proteins, some of the functions could acted by PARG itself. We selected most functions PARG had, and list some proteins which have the same functions with PARG. You can find most of the proteins on our site.
| Function | Related Protein |
|---|
Interacting Protein
PARG has direct interactions with proteins and molecules. Those interactions were detected by several methods such as yeast two hybrid, co-IP, pull-down and so on. We selected proteins and molecules interacted with PARG here. Most of them are supplied by our site. Hope this information will be useful for your research of PARG.
PARP1
Resources
Related Services
Related Products
References
- Larrea, SCV; Schlesinger, M; et al. Host Cell Poly(ADP-Ribose) Glycohydrolase Is Crucial for Trypanosoma cruzi Infection Cycle. PLOS ONE 8:-(2013).
- Gagne, JP; Pic, E; et al. Quantitative proteomics profiling of the poly(ADP-ribose)-related response to genotoxic stress. NUCLEIC ACIDS RESEARCH 40:7788-7805(2012).


