HSPA5
-
Official Full Name
heat shock 70kDa protein 5 (glucose-regulated protein, 78kDa) -
Overview
The HSP70 family is composed of four highly conserved proteins: HSP70, HSC70, GRP75 and GRP78. GRP78 is localized in the endoplasmic reticulum where it receives imported secretory proteins and is involved in the folding and translocation of nascent peptide chains. -
Synonyms
HSPA5;heat shock 70kDa protein 5 (glucose-regulated protein, 78kDa);GRP78, heat shock 70kD protein 5 (glucose regulated protein, 78kD);78 kDa glucose-regulated protein;BiP;immunoglobulin heavy chain-binding protein;endoplasmic reticulum lumenal Ca(2+)-binding protein grp78;MIF2;GRP78;FLJ26106
Recombinant Proteins
- Rhesus macaque
- Human
- Rat
- Zebrafish
- Mouse
- Chicken
- Mammalian Cell
- E.coli
- Human
- Wheat Germ
- HEK293T
- HEK293
- Yeast
- Baculovirus Insect Cells
- In Vitro Cell Free System
- Mammalian cells
- HEK293F
- His
- Non
- GST
- Myc&DDK
- His&Fc&Avi
- His&GST
- Flag
Background
What is HSPA5 protein?
HSPA5 gene (heat shock protein family A (Hsp70) member 5) is a protein coding gene which situated on the long arm of chromosome 9 at locus 9q33. The protein encoded by this gene is a member of the heat shock protein 70 (HSP70) family. This protein localizes to the lumen of the endoplasmic reticulum (ER) where it operates as a typical HSP70 chaperone involved in the folding and assembly of proteins in the ER and is a master regulator of ER homeostasis. During cellular stress, as during viral infection or tumorogenesis, this protein interacts with the transmembrane stress sensor proteins PERK (protein kinase R-like endoplasmic reticulum kinase), IRE1 (inositol-requiring kinase 1), and ATF6 (activating transcription factor 6) where it acts as a repressor of the unfolded protein response (UPR) and also plays a role in cellular apoptosis and senescence. Elevated expression and atypical translocation of this protein to the cell surface has been reported in viral infections and some types of cancer cells. At the cell surface this protein may facilitate viral attachment and entry to host cells. The HSPA5 protein is consisted of 654 amino acids and HSPA5 molecular weight is approximately 72.3 kDa.
What is the function of HSPA5 protein?
HSPA5 protein, also known as GRP78 or BiP, is a member of the heat shock protein 70 (HSP70) family localized in the endoplasmic reticulum lumen. As a typical HSP70 chaperone, it is involved in the folding and assembly of proteins in the endoplasmic reticulum and acts as the master regulator of endoplasmic reticulum homeostasis. HSPA5 proteins also have RNA-binding functions and may regulate gene expression through interaction with RNA. In addition, HSPA5 protein is expressed on the cell surface and may facilitate the binding and entry of viruses into host cells, such as Dengue virus, Zika virus, and Japanese encephalitis virus.
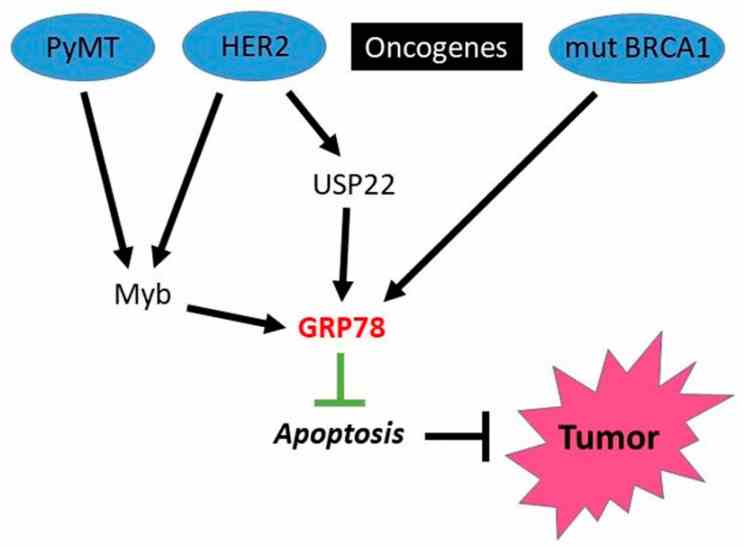
Fig1. Possible mechanisms of tumorigenesis mediated by intracellular GRP78. (Alexander E Kabakov, 2021)
HSPA5 Related Signaling Pathway
HSPA5 acts as a molecular chaperone to assist in the correct assembly of newly synthesized polymeric protein complexes in the ER, and is involved in the correct folding of proteins as well as the degradation of misfolded proteins. HSPA5 binding genes are involved in KEGG pathways such as cell cycle, RNA transport, and protein processing in the endoplasmic reticulum. The target genes of HSPA5 are enriched in the AMPK signaling pathway, which is a major regulatory factor in NAFLD and affects aging, inflammation, oxidation, lipid and glucose metabolism. HSPA5 can escape from the endoplasmic reticulum to the cell surface and control cell signaling, proliferation, apoptosis, and immune response under ER stress. HSPA5 could inhibit the process of iron death through P53/SLC7A11/GPX4 signaling pathway, and affect the sensitivity of tumor cells to iron death inducers.
HSPA5 Related Diseases
The expression level of HSPA5 in NAFLD is related to the disease status, and may be involved in the development of the disease by regulating the expression and splicing of NAFLD-related genes. HSPA5 was up-regulated in a variety of tumors, including hepatocellular carcinoma, breast cancer, and pancreatic cancer, and its high expression level was associated with tumor progression, invasiveness, and poor prognosis. HSPA5 may be involved in regulating protein folding and stress response in the cardiovascular system, and its abnormality may be related to the pathogenesis of some cardiovascular diseases. HSPA5 may act as host cell receptor for some virus infections and participate in the process of virus invasion and replication. It is also associated with neurological and immune-related diseases.
Bioapplications of HSPA5
HSPA5 has become a popular target for drug development due to its key role in a variety of diseases, especially in the field of cancer therapy, where drugs targeting HSPA5 may help inhibit the proliferation and invasion of tumor cells. The potential role of HSPA5 in non-alcoholic fatty liver disease (NAFLD) and other metabolic diseases makes it a novel strategy for the treatment of these diseases. The regulatory role of HSPA5 in immune response provides the possibility for the development of new therapies for autoimmune and inflammatory diseases. The interaction of HSPA5 with some viruses provides a new perspective for understanding the mechanism of virus infection and developing antiviral strategies.
Case Study
Case Study 1: Yongchang Qian, 2013
In this study, researchers explored the possibility that Cu forms a specific complex with Hspa5 by assaying stoichiometric binding of Cu and other metals to recombinant human HSPA5 (rh-HSPA5) in silico. Spectrophotometric analysis showed that incubation of rh-HSPA5 with Cu but not with Fe, Mn, Zn, or Pb in the presence of ascorbic acid produced an absorbance peak at 470 nm. Furthermore, the absorbance peak was absent when bovine serum albumin was incubated with Cu and when another recombinant protein YWHAZ-14-3-3-Zeta carrying a 6× histidine tag identical to the tag in the rh-HSPA5 was incubated with Cu. The absorbance peak produced by Cu and rh-HSPA5 was abolished by EDTA treatment and was stabilized at pH levels above 6.5. Assay of the stoichiometry of metal binding to the purified rh-HSPA5 showed that one molecule of the rh-HSPA5 could chelate 1 or 2 Cu, 13 iron (Fe), 5 zinc (Zn) and 10 lead (Pb) ions but not manganese (Mn).

Fig1. Total bacterial proteins were analyzed by a 10 % SDS-PAGE gel.

Fig2. pH stability of Cu-rh-HSPA5 complexes.
Case Study 2: Takeshi Ijuin, 2016
Skeletal muscle and kidney-enriched inositol polyphosphate phosphatase (SKIP), a PIP3 phosphatase, has been implicated in the regulation of insulin signaling in skeletal muscle. SKIP interacts with Pak1 and glucose-regulated protein 78 (GRP78), both of which are necessary for the regulation of insulin signaling. In this study, researchers showed that GRP78 directly binds to the SKIP C-terminal homology (SKICH) domain of SKIP and that this binding is necessary for the localization of SKIP at the ER. In addition, in vitro binding analysis showed that GRP78 and Pak1 competitively bind to SKIP. Taken together, these findings suggest a model by which GRP78 regulates intracellular localization of SKIP and how SKIP binds to Pak1 on insulin stimulation.

Fig3. Direct binding between GRP78 and SKIP.

Fig4. BIAcore analysis of immobilized SKIP binding to the GRP78 protein.
Quality Guarantee
.
.jpg)
Fig1. SDS-PAGE (HSPA5-5107H)
.
.jpg)
Fig2. SDS-PAGE (HSPA5-3349H)
Involved Pathway
HSPA5 involved in several pathways and played different roles in them. We selected most pathways HSPA5 participated on our site, such as Protein export,Protein processing in endoplasmic reticulum,Antigen processing and presentation, which may be useful for your reference. Also, other proteins which involved in the same pathway with HSPA5 were listed below. Creative BioMart supplied nearly all the proteins listed, you can search them on our site.
| Pathway Name | Pathway Related Protein |
|---|---|
| Protein export | IMMP1L,SRP14,SEC63,SRP19,ARXES1,SRPRB,SEC11C,SRP68,OXA1L,SRP72 |
| Protein processing in endoplasmic reticulum | UBQLN1,NSFL1C,CAPN2A,SAR1AB,UBE2G1B,UBQLNL,CUL1B,HSPA6,CALRL2,MARCH6 |
| Prion diseases | Hc,NCAM2,CCL5,MAPK1,STIP1,MAP2K1,C8G,FYN,MAP2K2,PRKACB |
| Antigen processing and presentation | TAP1,RFXAP,HSPA2,KLRC2,LGMN,HLA-E,Ctsl,HLA-DOB,HLA-DRB3,KIR2DS5 |
| Thyroid hormone synthesis | ATP1B2,ATP1A4,PRKACB,ITPR3,PRKACG,TPO,ASGR1,GPX8,TSHR,DUOXA2 |
Protein Function
HSPA5 has several biochemical functions, for example, ATP binding,ATPase activity,calcium ion binding. Some of the functions are cooperated with other proteins, some of the functions could acted by HSPA5 itself. We selected most functions HSPA5 had, and list some proteins which have the same functions with HSPA5. You can find most of the proteins on our site.
| Function | Related Protein |
|---|---|
| glycoprotein binding | SHB,FBXO17,RGMA,STX1A,Shh,VIM,DNAJC5,IDE,LRRK2,SELP |
| ribosome binding | SEC61A1,BAG6,CPEB1B,CPEB2,ETF1,MTRF1L,SEC61B,ETF1B,IGHMBP2,NAA16 |
| chaperone binding | CLU,DNAJB2,SOD1,TG,PFDN6,BAG3,BAX,DNAJA3A,PFDN4,SLC25A17 |
| unfolded protein binding | CDC37,NPM1,LMAN1,PFDN6,CCT6B,SSUH2.2,TAPBP,HSPE1,PFDN2,DNAJA2 |
| protein domain specific binding | ARHGEF4,TBL1X,VAPA,HOXB1,CNTLN,NR0B2,E2F4,CALM1,TNFAIP1,FOXA3 |
| misfolded protein binding | SDF2L1,DNAJC10,F12,DNAJC3,HSPD1,HDAC6,BAG6,STUB1,EDEM1,DNAJB9 |
| protein binding | SULT1B1,APTX,SOX6,RABL2A,C10orf96,COPS7B,SLC8A1,KSR1,TUBB4A,IL4R |
| ATP binding | SPO11,UBE2L3,ALDH18A1,PTK2BB,RLN1,DDX18,Fert2,EHD3,STK10,KIF11 |
| enzyme binding | PHB,APBA3,PLCE1,SPDL1,NOXO1,GSTM4,HIST1H2AM,PRMT1,TNKS2,HIST1H2AG |
Interacting Protein
HSPA5 has direct interactions with proteins and molecules. Those interactions were detected by several methods such as yeast two hybrid, co-IP, pull-down and so on. We selected proteins and molecules interacted with HSPA5 here. Most of them are supplied by our site. Hope this information will be useful for your research of HSPA5.
DNAJB11;DMKN;RAF1;Pawr;PAWR
Resources
Related Services
Related Products
References
- Negroni, L; Taouji, S; et al. Integrative Quantitative Proteomics Unveils Proteostasis Imbalance in Human Hepatocellular Carcinoma Developed on Nonfibrotic Livers. MOLECULAR & CELLULAR PROTEOMICS 13:3473-3483(2014).
- Maetzler, W; Apel, A; et al. Comparable Autoantibody Serum Levels against Amyloid- and Inflammation-Associated Proteins in Parkinson's Disease Patients and Controls. PLOS ONE 9:-(2014).


