FUT8
-
Official Full Name
fucosyltransferase 8 (alpha (1,6) fucosyltransferase) -
Overview
This gene encodes an enzyme belonging to the family of fucosyltransferases. The product of this gene catalyzes the transfer of fucose from GDP-fucose to N-linked type complex glycopeptides. This enzyme is distinct from other fucosyltransferases which catalyze alpha1-2, alpha1-3, and alpha1-4 fucose addition. The expression of this gene may contribute to the malignancy of cancer cells and to their invasive and metastatic capabilities. Alternative splicing results in multiple transcript variants. -
Synonyms
FUT8;fucosyltransferase 8 (alpha (1,6) fucosyltransferase);alpha-(1,6)-fucosyltransferase;6)-fucosyltransferase;6-fucosyltransferase;Alpha (1,6) fucosyltransferase;Alpha-(1;Alpha1 6FucT;alpha1-6FucT;Fucosyltransferase 8;FUT8_HUMAN;GDP fucose glycoprotein fucosyltransferase;GDP L Fuc:N acetyl beta D glucosaminide alpha1,6 fucosyltransferase;GDP-fucose--glycoprotein fucosyltransferase;GDP-L-Fuc:N-acetyl-beta-D-glucosaminide alpha1;Glycoprotein 6 alpha L fucosyltransferase;Glycoprotein 6-alpha-L-fucosyltransferase;MGC26465;GDP-L-Fuc:N-acetyl-beta-D-glucosaminide alpha1,6-fucosyltransferase
Recombinant Proteins
- Human
- Chicken
- Mouse
- Rhesus macaque
- Rat
- Hamster
- Sus scrofa (Pig)
- Insect Cell
- CHO
- Mammalian Cell
- E.coli
- Wheat Germ
- HEK293
- In Vitro Cell Free System
- HEK293T
- E.coli expression system
- His
- Non
- His&Fc&Avi
- Myc&DDK
- His&GFP
- GST
Background
What is FUT8 Protein?
FUT8 gene (fucosyltransferase 8) is a protein coding gene which situated on the long arm of chromosome 14 at locus 14q23. This gene encodes an enzyme belonging to the family of fucosyltransferases. The product of this gene catalyzes the transfer of fucose from GDP-fucose to N-linked type complex glycopeptides. This enzyme is distinct from other fucosyltransferases which catalyze alpha1-2, alpha1-3, and alpha1-4 fucose addition. The expression of this gene may contribute to the malignancy of cancer cells and to their invasive and metastatic capabilities. The FUT8 protein is consisted of 575 amino acids and FUT8 molecular weight is approximately 66.5 kDa.
What is the Function of FUT8 Protein?
FUT8 protein is an alpha (1,6) -fucosyltransferase whose primary physiological function is to catalyze the transfer of fucose to the n-acetylglucosamine (GlcNAc) -linked portion during n-glucose chain synthesis, which is a common feature of the n-glucose chain core structure. Its abnormal expression can interfere with the normal function of tumor signaling pathway, leading to malignant changes such as cell proliferation, invasion, metastasis and immunosuppression. In addition, FUT8 is also involved in homeostasis maintenance of the tumor microenvironment.

Fig1. Synthesis pathway of FUT8 donor in humans. (Meng Shi, 2024)
FUT8 Related Signaling Pathway
In colorectal cancer, FUT8 may influence cancer cell proliferation and invasion through the AKT/ beta-catenin signaling pathway. In osteosarcoma, decreased FUT8 expression is associated with tumor growth and progression. FUT8 affects the survival strategy of OS by altering the level of core fucosylation of TNF receptors. FUT8 drives proliferation and invasion of trophoblast cells through the IGF-1/IGF-1R signaling pathway. We found that FUT8 modifies the α1, 6-fucosylation of IGF-1R and regulates IGF-1-dependent activation of IGF-1R, MAPK, and PI3K/Akt signaling pathways. In osteosarcoma, downregulation of FUT8 leads to activation of the TNF/NF-κB2 signaling pathway, thereby blocking the mitochondria-dependent apoptosis process.
FUT8 Related Diseases
FUT8 protein is abnormally expressed in a variety of cancers, including breast cancer, colon cancer, and stomach cancer. Studies have shown that abnormal expression of FUT8 protein can promote the growth and metastasis of tumor cells. The FUT8 protein also plays an important role in autoimmune diseases. For example, the expression level of FUT8 protein in synovial tissue of joints in patients with rheumatoid arthritis is significantly increased. The FUT8 protein has also been linked to inflammatory diseases. The FUT8 protein also plays a role in neurodegenerative diseases. For example, the expression level of the FUT8 protein is significantly reduced in the brain tissue of Alzheimer's patients.
Bioapplications of FUT8
Abnormal expression of the FUT8 protein is associated with a variety of diseases, especially in a variety of cancers where it is often upregulated. Therefore, the FUT8 protein can be used as a biomarker for these diseases. For example, while core fucose-modified alpha-fetoprotein (AFP) is an important biomarker for hepatocellular carcinoma, and its expression is also elevated in some benign liver diseases, the core fucose-modified AFP is only elevated in hepatocellular carcinoma, and thus can be used as a more reliable biomarker for hepatocellular carcinoma. The FUT8 protein is a target for drug development, especially in the field of tumor therapy.
Case Study
Case Study 1: Irina Dyukova, 2021
The patterns of glycosylation in monoclonal antibodies (mAbs) are subject to considerable variation depending on the host cell line used, which can in turn influence the safety, effectiveness, and potential to provoke an immune response of the mAbs. It has been shown that different arrangements of terminal galactose on the Man α1-3 or Man α1-6 arms of glycans can alter the effector functions and structural dynamics of mAbs. Therefore, establishing a reliable method to differentiate the positional isomers of these glycans is essential for ensuring the quality of mAbs. In this study, researchers utilize high-resolution ion mobility in conjunction with cryogenic infrared spectroscopy to identify glycan isomers with distinct terminal galactose positions, taking G1F as a model. By employing selective enzymatic synthesis, we are able to specifically produce the G1(α1-6)F isomer, which assists in the precise assignment of peaks in arrival-time distributions and their corresponding infrared spectra. This research underscores the effectiveness of our approach in analyzing the glycosylation of mAbs.

Fig1. Positional isomers of G-NGA2 and G1F glycans with a scheme for chemoenzymatic synthesis of the G1(α1-6)F isomer using human α1,6-fucosyltransferase FUT8.
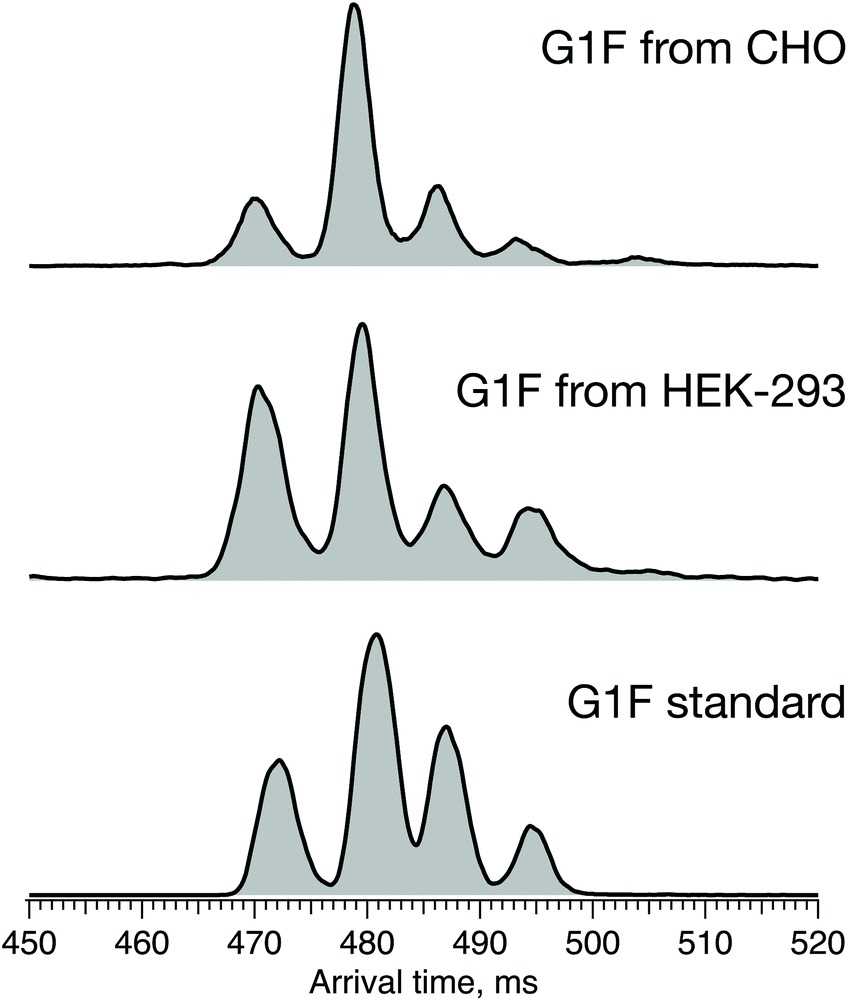
Fig2. ATDs of G1F standard (bottom) and G1F released from recombinant IgG antibody produced in HEK-293 (middle) and CHO cell lines (top) after 60 m SLIM-IMS separation.
Case Study 2: Seita Tomida, 2022
FUT8, like numerous other glycosyltransferases, functions as a type-II membrane protein with its extensive C-terminal catalytic domain connected to the FUT8 stem region, which includes two α-helices. Despite the known role of stem regions in the Golgi apparatus localization of glycosyltransferases, the specific role of FUT8's stem region remains undefined. This research has discovered that this stem region is crucial for the oligomerization of the FUT8 enzyme. Researchers introduced a mutation, FUT8Δstem, in which the stem region was substituted with glycine/serine linkers, into FUT8-knockout HEK293 cells. Through immunoprecipitation and native-PAGE analysis, they observed that while the wild-type FUT8 formed multimeric structures, the FUT8Δstem mutation disrupted this multimerization within the cellular context, even though the mutants maintained their specific activity. Moreover, the mutated protein exhibited reduced steady-state levels, increased localization to the endoplasmic reticulum, and a shorter lifespan compared to the wild-type FUT8, indicating that the absence of the stem region led to the destabilization of the FUT8 protein. Further immunoprecipitation analysis of a mutant missing a segment of the stem region indicated that the first α-helix in the FUT8 stem region is essential for the formation of multimers.
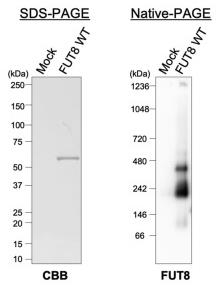
Fig3. Soluble His-FUT8 was purified from COS7 culture medium using a Ni2+ column.

Fig4. Interaction between RPN1 and FUT8 WT, Δstem-GSx22, or Δstem-GSx40.
Quality Guarantee
High Purity
.jpg)
Fig1. SDS-PAGE (FUT8-4567H)
.
.jpg)
Fig2. SDS-PAGE (FUT8-6301H)
Involved Pathway
FUT8 involved in several pathways and played different roles in them. We selected most pathways FUT8 participated on our site, such as N-Glycan biosynthesis,Glycosaminoglycan biosynthesis - keratan sulfate,Metabolic pathways, which may be useful for your reference. Also, other proteins which involved in the same pathway with FUT8 were listed below. Creative BioMart supplied nearly all the proteins listed, you can search them on our site.
| Pathway Name | Pathway Related Protein |
|---|---|
| Transcriptional misregulation in cancer | SP1,ATM,RUNX2,DOT1L,ITGB7,ASPSCR1,TLX3,HIST1H3F,Pdgfa&Pdgfb,KDM6A |
| Metabolic pathways | CBS,HSD17B12B,PGD,NDUFB4,LPCAT4,PLD2,HK1,MAT2AA,UGT8,TPH1A |
| N-Glycan biosynthesis | ALG10,RABGNT1,ALG13,MAN2A1,RPN1,MAN1B1,ALG3,MAN1A2,DPAGT1,ST6GAL2A |
| Glycosaminoglycan biosynthesis - keratan sulfate | ST3GAL3,B3GNT1,CHST2A,B4GALT3,CHST6,CHST4,B3GNT2B,B4GALT4,ST3GAL2,B4GALT2 |
Protein Function
FUT8 has several biochemical functions, for example, SH3 domain binding,glycoprotein 6-alpha-L-fucosyltransferase activity. Some of the functions are cooperated with other proteins, some of the functions could acted by FUT8 itself. We selected most functions FUT8 had, and list some proteins which have the same functions with FUT8. You can find most of the proteins on our site.
| Function | Related Protein |
|---|---|
| SH3 domain binding | ELMO3,ENKUR,ERRFI1,ITGB1BP2,SH3BP5LA,ARHGAP27,SH3BGRL,MAPK15,DOCK4,SH3BGRL2 |
| glycoprotein 6-alpha-L-fucosyltransferase activity | FUT8A |
Interacting Protein
FUT8 has direct interactions with proteins and molecules. Those interactions were detected by several methods such as yeast two hybrid, co-IP, pull-down and so on. We selected proteins and molecules interacted with FUT8 here. Most of them are supplied by our site. Hope this information will be useful for your research of FUT8.
env;dAK1_1
Resources
Related Services
Related Products
References
- Li, WZ; Yu, R; et al. Core Fucosylation of IgG B Cell Receptor Is Required for Antigen Recognition and Antibody Production. JOURNAL OF IMMUNOLOGY 194:2596-2606(2015).
- Wang, YQ; Fukuda, T; et al. Loss of alpha 1,6-fucosyltransferase suppressed liver regeneration: implication of core fucose in the regulation of growth factor receptor-mediated cellular signaling. SCIENTIFIC REPORTS 5:-(2015).



