DNASE1
-
Official Full Name
deoxyribonuclease I -
Overview
This gene encodes a member of the DNase family. This protein is stored in the zymogen granules of the nuclear envelope and functions by cleaving DNA in an endonucleolytic manner. At least six autosomal codominant alleles have been characterized, DNASE1*1 through DNASE1*6, and the sequence of DNASE1*2 represented in this record. Mutations in this gene have been associated with systemic lupus erythematosus (SLE), an autoimmune disease. A recombinant form of this protein is used to treat the one of the symptoms of cystic fibrosis by hydrolyzing the extracellular DNA in sputum and reducing its viscosity. Alternate transcriptional splice variants of this gene have been observed but have not been thoroughly characterized. -
Synonyms
DNASE1;deoxyribonuclease I;DNL1;deoxyribonuclease-1;Dornase alfa;Deoxyribonuclease 1;Deoxyribonuclease1;DeoxyribonucleaseI;DNASE 1;DNase I lysosomal;DNaseI;DNL 1;DRNI;FLJ38093;Human urine deoxyribonuclease I;DNase I, lysosomal
Recombinant Proteins
- Human
- Bovine
- Rat
- Mouse
- Chicken
- Zebrafish
- Cynomolgus
- E.coli
- HEK293
- Bovine
- Mammalian Cell
- Human Cell
- Wheat Germ
- CHO
- CHO Cell
- In Vitro Cell Free System
- Pancreas
- GST
- His
- Non
- Fc
- His&GST
- His&Fc
- His&Fc&Avi
Background
What is DNASE1 protein?
DNASE1 (deoxyribonuclease 1) gene is a protein coding gene which situated on the short arm of chromosome 16 at locus 16p13. DNASE1 is a nonspecific endonuclease capable of hydrolyzing single - or double-stranded DNA to produce mono - and oligo - deoxynucleotides containing 5' -phosphate groups and 3' -Oh groups. The optimal working pH range of this enzyme is 7-8, its activity is dependent on Ca2+, and can be activated by other bivalent metal ions such as Mn2+, Mg2+, Zn2+, etc. The DNASE1 protein is consisted of 282 amino acids and its molecular mass is approximately 31.4 kDa.
What is the function of DNASE1 protein?
DNASE1 is an enzyme that performs important biological functions in the human body. It acts primarily on DNA molecules and is able to hydrolyze single - and double-stranded DNA by cutting phosphodiester bonds to produce oligonucleotides with 5' -phosphate groups and 3' -hydroxyl ends. The activity of DNASE1 is dependent on calcium ions and can be activated by other bivalent cations such as magnesium and manganese. This enzyme has important roles both inside and outside the cell, including participating in the process of DNA degradation and repair, maintaining genomic stability, and clearing DNA outside the cell to prevent autoimmune reactions. In addition, DNASE1 also plays a role in cell death and apoptosis by partially degrading genomic DNA as a positive control for apoptosis.
DNASE1 Related Signaling Pathway
By interacting with pattern recognition receptors, DNASE1 is involved in the recognition of pathogen-associated molecular patterns on the surface of pathogenic microorganisms, thereby initiating an innate immune response. In addition, DNASE1 is involved in the regulation of the NF-κB signaling pathway, which is essential for the production of inflammatory cytokines and type I interferons against pathogenic microbial infections. In the non-classical NF-κB signaling pathway, DNASE1 can promote the phosphorylation of NIK, thereby inhibiting the activation of this pathway. In addition, DNASE1 is also associated with interferon signaling pathway and regulates signaling pathway conduction by interacting with other proteins, such as TRAF3, TRIF, MAVS, STING, etc. In the c-GAS signaling pathway, DNASE1 is involved in sensing DNA that should not be present in the cytoplasm, activating STING protein, and then promoting the activation of TBK1 and IRF3, inducing the expression of type I interferon and other immune response genes.
DNASE1 Related Diseases
DNASE1 is known to be associated with Systemic Lupus Erythematosus (SLE), an autoimmune disease characterized by the body's immune system mistakenly attacking its own tissues and organs. In addition, DNASE1 has been associated with ischemic stroke, coronary artery disease, asthma, acute kidney injury, graft-versus-host disease, sarcoidosis, amyotrophic lateral sclerosis, encephalitis, and other autoimmune diseases. In these diseases, abnormal expression or function of DNASE1 may affect key biological processes such as inflammatory response, immune regulation, and tissue repair. For example, in studies of non-alcoholic fatty liver disease (NAFLD), depletion of neutrophil extracellular traps (NETs) by using DNase 1 was found to alleviate liver inflammation and fibrosis, suggesting that DNASE1 plays a role in regulating NETs and the clotting system, which in turn affects disease progression.
Bioapplications of DNASE1
In laboratory studies, DNase I is primarily used to remove DNA contamination from RNA samples, which is essential for performing experiments such as reverse transcription PCR (RT-PCR) or RNA sequencing to ensure the accuracy of the results. In addition, DNase I is used in footprinting analysis, a technique that identifies the binding sites of DNA-binding proteins on DNA molecules, revealing protein-binding sequences by enzymically cutting regions of DNA that are not protected by protein binding. DNase I is also used in DNA labeling techniques, such as notch translation, which is a method of introducing labeled nucleotides onto a DNA strand and is often used in the preparation of DNA probes.
In terms of clinical application, DNase I has been approved for the treatment of pulmonary cystic fibrosis, which is administered by inhalation to help reduce sticky secretions and reduce the occurrence of infections. In addition, DNase I and DNase1L3 also play a role in systemic lupus erythematosus (SLE) and other inflammation-related diseases, where they have potential therapeutic value in regulating the immune system and reducing inflammatory responses. DNase I's GMP grade products can also be used in pharmaceutical production, ensuring quality and safety standards in the manufacturing process.
Case Study
Case Study 1: Yujia Xia, 2020
Liver metastasis is the main cause of colorectal cancer (CRC)-related death. Neutrophil extracellular traps (NETs) play important roles in CRC progression. Deoxyribonuclease I (DNase I) has been shown to alter NET function by cleaving DNA strands comprising the NET backbone. Moreover, DNase I displays high antimetastatic activity in multiple tumor models. To circumvent long-term daily administrations of recombinant DNase I, the researchers have developed an adeno-associated virus (AAV) gene therapy vector to specifically express DNase I in the liver. This study demonstrates AAV-mediated DNase I liver gene transfer following a single intravenous injection suppresses the development of liver metastases in a mouse model of CRC liver metastasis. Neutrophil infiltration and NET formation were inhibited in tumor tissues with AAV-DNase I treatment. This approach restored local immune responses at the tumor site by increasing the percentage of CD8+ T cells while keeping CD4+ T cells similar between AAV-DNase I and AAV-null treatments.

Fig1. DNase I levels were determined at 24–72 h post-transduction with different AAV vector concentrations in HepG2 cells.
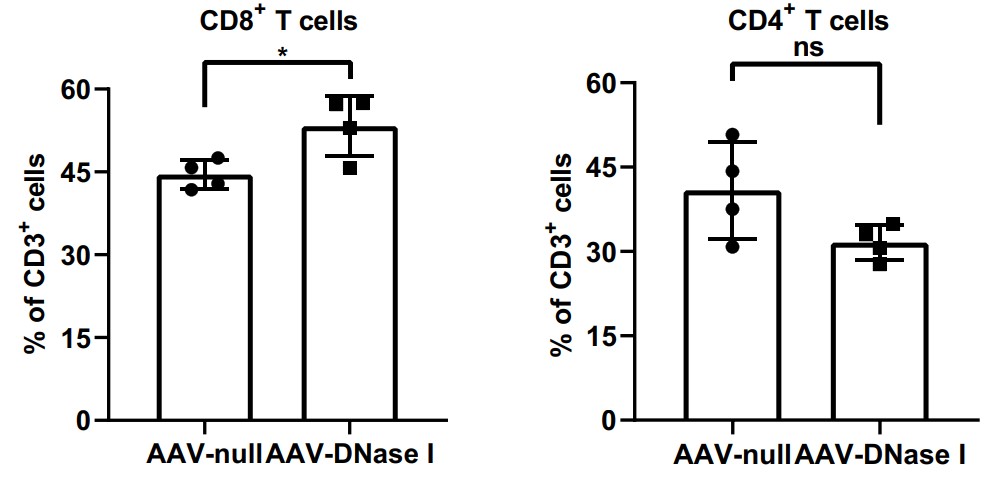
Fig2. Quantification of cellular percentages in tumor tissues by flow cytometry.
Case Study 2: Paul T King, 2017
This study aims to investigate if deoxyribonuclease (DNase) 1 is a potential therapeutic agent to reduce pathogenic effects of cigarette smoke exposure in the lung. Cigarette smoke causes protease imbalance with excess production of proteases, which is a key process in the pathogenesis of emphysema. The mechanisms responsible for this effect are not well-defined. These studies demonstrate both in vitro and in vivo that cigarette smoke significantly increases the expression of neutrophil and macrophage extracellular traps with coexpression of the pathogenic proteases, neutrophil elastase and matrix metalloproteinases 9 and 12. This response to cigarette smoke was significantly reduced by the addition of DNase 1, which also significantly decreased macrophage numbers and lung proteolysis. DNase 1, a treatment currently in clinical use, can diminish the pathogenic effects of cigarette smoke.
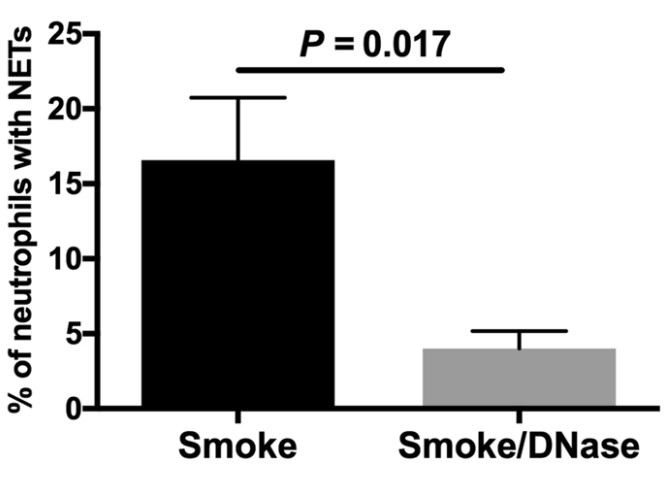
Fig3. The addition of DNase 1 reduces the expression of cigarette smoke-induced NETs.
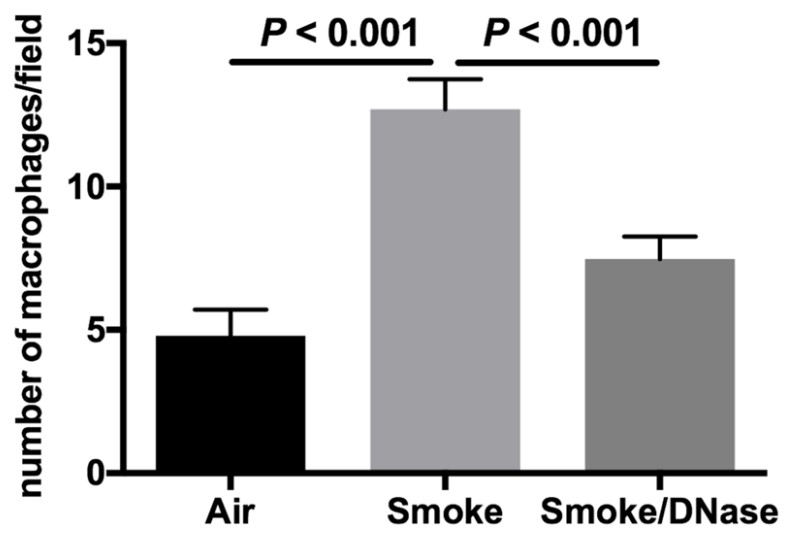
Fig4. The addition of DNase 1 reduces macrophage numbers.
Quality Guarantee
High Purity
.jpg)
Fig1. SDS-PAGE (DNASE1-2774H)
.
.jpg)
Fig2. SDS-PAGE (DNASE1-1529H)
Involved Pathway
DNASE1 involved in several pathways and played different roles in them. We selected most pathways DNASE1 participated on our site, such as , which may be useful for your reference. Also, other proteins which involved in the same pathway with DNASE1 were listed below. Creative BioMart supplied nearly all the proteins listed, you can search them on our site.
| Pathway Name | Pathway Related Protein |
|---|
Protein Function
DNASE1 has several biochemical functions, for example, actin binding,deoxyribonuclease I activity,endodeoxyribonuclease activity. Some of the functions are cooperated with other proteins, some of the functions could acted by DNASE1 itself. We selected most functions DNASE1 had, and list some proteins which have the same functions with DNASE1. You can find most of the proteins on our site.
| Function | Related Protein |
|---|---|
| protein binding | GPM6A,PSMD3,SYCE3,CDH23,RGP1,YIPF3,PRDM10,COL6A2,C12orf43,ALPP |
| endodeoxyribonuclease activity | ERCC4,DNASE1L3,DNASE1L2,ERCC5,APLF,RPS3,DNASE2A,APEX1,ZRANB3,MBD4 |
| actin binding | TPM4,SMYHC3,ABLIM3,GC,MYH1B,SHROOM1,SMTNL,MYO10,SNTB1,EMD |
| deoxyribonuclease I activity | DICER1 |
Interacting Protein
DNASE1 has direct interactions with proteins and molecules. Those interactions were detected by several methods such as yeast two hybrid, co-IP, pull-down and so on. We selected proteins and molecules interacted with DNASE1 here. Most of them are supplied by our site. Hope this information will be useful for your research of DNASE1.
Resources
Related Services
Related Products
References
- Vogel, B; Frantz, S; et al. Determination of DNase activity by degradation of ethidium bromide-DNA complexes using a fluorescence plate reader. ANALYTICAL BIOCHEMISTRY 471:73-79(2015).
- Fragoulis, GE; Vakrakou, AG; et al. Impaired degradation and aberrant phagocytosis of necrotic cell debris in the peripheral blood of patients with primary Sjogren's syndrome. JOURNAL OF AUTOIMMUNITY 56:12-22(2015).


