Primordial Germ Cell Markers
Related Symbol Search List
Immunology Background
Background
Primordial germ cells (PGCs) are embryonic stem cells that give rise to gametes, or eggs and sperm, in vertebrate embryos.
PGCs are oval or round in shape, with large nuclei and irregular contours. They are characterized by their high alkaline phosphatase activity. PGCs originate outside the embryo early in development, from a small population of epiblast cells set aside before gastrulation. They then migrate across the embryo to the genital ridges, which will form the ovaries in females and the testes in males, in a three-phase process called separation, migration, and colonization.
Once they reach the gonads, PGCs undergo mitotic proliferation, then meiosis, and differentiate into mature gametes. Proper PGC specification, development, and sexual identity are fundamental for fertility and the generational inheritance of traits. For example, in the absence of the Sry gene, PGCs differentiate into eggs, while in the presence of Sry, they differentiate into sperm.
PGCs require both an intrinsic motility program and external guidance cues to survive and migrate successfully. These cues are mediated by protein and lipid signaling and involve both attractive and repulsive signals.
Roles of Primordial Germ Cell
- Gamete Production: PGCs are the foundation for the production of sperm and eggs, the key components of sexual reproduction.
- Genetic Continuity: PGCs carry genetic information from one generation to the next, ensuring the continuity of the species.
- Gonadal Development: PGCs play a pivotal role in the development of the gonads, where they undergo further differentiation into spermatozoa or ova.
- Sex Determination: PGCs contribute to sexual development and help establish the individual's sex characteristics.
 Fig.1 Development of primordial germ cells (PGCs) into primordial follicles in mammalian ovaries. (Hernández-Ochoa, et al., 2016)
Fig.1 Development of primordial germ cells (PGCs) into primordial follicles in mammalian ovaries. (Hernández-Ochoa, et al., 2016)Primordial germ cell (PGC) markers are specific molecules or proteins that are uniquely expressed in primordial germ cells during early embryonic development.
Role of Primordial Germ Cell Markers
- Identification and Isolation: PGC markers are utilized to distinguish primordial germ cells from other cell types during embryogenesis, allowing researchers to specifically target and study these cells.
- Tracking PGC Development: By observing the expression patterns of PGC markers, scientists can track the development and migration of primordial germ cells throughout embryogenesis, providing insights into their differentiation processes.
- Characterization of Developmental Stages: The expression of specific PGC markers can indicate different developmental stages of primordial germ cells, aiding in understanding the progression of germ cell development.
- Study of Germ Cell Fate: Primordial germ cell markers help researchers investigate the fate and function of germ cells, including their role in gametogenesis and reproductive processes.
- Insights into Regulatory Mechanisms: By studying the regulation of PGC markers, researchers can uncover the molecular mechanisms that govern germ cell specification, migration, and differentiation.
- Potential Clinical Applications: Understanding primordial germ cell markers can have implications for fertility research, reproductive technologies, and potential treatments for reproductive disorders.
- Comparative Studies: Comparing the expression of PGC markers across different species can provide insights into evolutionary aspects of germ cell development and reproductive strategies.
Overall, primordial germ cell markers are valuable tools in reproductive biology research, offering a means to explore the intricate processes of germ cell development, fertility, and genetic inheritance.
Common Primordial Germ Cell Markers
| PGC Markers | Details |
|---|---|
| Stella (Dppa3) | Stella is a well-established marker for PGCs and is crucial for maintaining their identity and function during development. |
| Octamer-binding transcription factor 4 (Oct4/POU5F1) | Oct4 is a key transcription factor that helps regulate PGC specification and pluripotency. |
| Fragilis (Ifitm3) | Fragilis is involved in germ cell development and migration and is used as a marker for PGCs. |
| Dead end (Dnd1) | Dead end is an essential RNA-binding protein for germ cell development and is commonly used to identify PGCs. |
| Blimp1 (Prdm1) | Blimp1 is a transcriptional repressor important for germ cell specification and maintenance. |
| C-Kit (CD117) | C-Kit is a receptor tyrosine kinase expressed in PGCs that plays a role in germ cell survival and migration. |
| Vasa (DDX4) | Vasa is a conserved germline marker essential for germ cell development and is expressed in PGCs. |
| Dazl (Deleted in azoospermia-like) | Dazl is an RNA-binding protein crucial for germ cell development and is commonly found in PGCs. |
| FUT4 (Fucosyltransferase 4) | FUT4 is important in the modification of glycoproteins and glycolipids. It may have roles in cell adhesion and signaling processes during germ cell development. |
| IFITM1, IFITM2, IFITM3 (Interferon-Induced Transmembrane Proteins 1, 2, 3): | These proteins are involved in the immune response to viral infections. |
| NANOS2 (Nanos C2HC-Type Zinc Finger 2) | NANOS2 is a key regulator of germ cell development in males. It plays a role in maintaining the undifferentiated state of germ cells and promoting spermatogenesis. |
| POU2F1 (POU Class 2 Homeobox 1) | POU2F1 is a transcription factor involved in regulating gene expression. |
| PRDM14 (PR Domain Containing 14) | PRDM14 is involved in germ cell specification and early embryonic development. It plays a role in maintaining pluripotency and regulating germ cell fate decisions. |
These markers serve as valuable tools for studying PGC development, tracking their migration, and understanding the molecular mechanisms that govern germ cell specification and differentiation. Their expression patterns provide insights into the intricate processes underlying reproductive biology and fertility.
 Fig.2 Human iPS cells differentiate into primordial germ cells (PGCs) and further differentiate into meiotic cells and the markers that have been identified at each stage. (Ishii T, et al., 2014)
Fig.2 Human iPS cells differentiate into primordial germ cells (PGCs) and further differentiate into meiotic cells and the markers that have been identified at each stage. (Ishii T, et al., 2014)Primordial Germ Cell Markers in Each Disease
Primitive germ cell markers play a crucial role in various diseases, as they help to identify and characterize cancer cells originating from primitive germ cells. These markers are proteins expressed on the surface of cancer cells that indicate their origin from germ cells. Here are some examples of how primitive germ cell markers are involved in specific diseases:
Testicular Cancer
Testicular cancer is a type of cancer that develops in the testicles, which are primitive germ cells that give rise to sperm. The most common type of testicular cancer is germ cell tumors, which can be further classified into seminomas and non-seminomas.
OCT4 (POU5F1), NANOG, SOX2, PLAP (Placental Alkaline Phosphatase), and AFP (Alpha-Fetoprotein) are used for the diagnosis, classification, and prognostication of TGCTs. For instance, OCT4, NANOG, and SOX2 are key markers of pluripotency and are often overexpressed in TGCTs. PLAP and AFP are used as diagnostic markers for certain subtypes of TGCTs. CD30 and PLAP are often used to diagnose and differentiate between these different types of testicular germ cell tumors (TGCTs).
Ovarian Cancer
Ovarian cancer can also originate from germ cells in the ovaries. Germ cell tumors account for a small percentage of ovarian cancers and are more common in younger women.
OCT4 (POU5F1), SOX2, GDF3 (Growth Differentiation Factor 3), c-KIT (CD117), and AFP are utilized in the diagnosis and characterization of ovarian germ cell tumors. OCT4, SOX2, and GDF3 are commonly expressed in these tumors, while c-KIT is a marker associated with certain subtypes. AFP elevation may indicate yolk sac tumors.
Extragonadal Germ Cell Tumors
In some cases, germ cell tumors can develop outside of the testicles or ovaries, in locations such as the mediastinum, pineal gland, or retroperitoneum. Primitive germ cell markers play a crucial role in diagnosing and classifying these extragonadal germ cell tumors.
OCT4 (POU5F1), CD30 (TNFRSF8), PLAP, hCG (Human Chorionic Gonadotropin), and AFP are used to identify and diagnose extragonadal germ cell tumors, which can occur in various locations outside the gonads. OCT4 and PLAP are commonly expressed, while hCG and AFP levels can help monitor tumor progression.
Mixed Germ Cell Tumors
Mixed germ cell tumors contain a combination of different germ cell components. Markers like OCT4, AFP, hCG, and PLAP are used to identify the different germ cell elements within these tumors.
Germ Cell Aplasia (Spermatogenic Failure)
DAZ (Deleted in Azoospermia), DAZL (Deleted in Azoospermia-Like), and VASA (DDX4) are associated with spermatogenic failure or germ cell aplasia, conditions where the development of spermatozoa is impaired. Mutations in these genes can lead to infertility due to defects in germ cell development.
Importance of Primitive Germ Cell Markers in Developmental Biology
Identification and Isolation of Primordial Germ Cells (PGCs)
Primitive germ cell markers are crucial for identifying and isolating PGCs from the developing embryo. This enables researchers to study these cells specifically and understand their unique characteristics and functions.
Tracking Germ Cell Development
By using primitive germ cell markers, researchers can track the developmental trajectory of germ cells from their origin to their differentiation into mature gametes. This provides insights into the complex processes involved in germ cell development.
Understanding Germ Cell Fate Determination
The expression patterns of primitive germ cell markers help in deciphering the molecular mechanisms that determine the fate of germ cells, including their differentiation into sperm or eggs. This knowledge is fundamental for understanding reproductive biology.
Characterization of Pluripotency and Differentiation
Primitive germ cell markers, such as OCT4 (POU5F1) and NANOG, are also markers of pluripotency. Studying these markers in the context of germ cells provides valuable information about the balance between pluripotency and differentiation during development.
Insights into Reproductive Disorders and Infertility
Aberrant expression or mutations in primitive germ cell markers can be associated with reproductive disorders and infertility. Studying these markers can help in diagnosing and understanding the underlying causes of such conditions.
Evolutionary Studies
Comparative analysis of primitive germ cell markers across species provides insights into the evolutionary conservation of germ cell development processes. This comparative approach helps in understanding the diversity and commonalities in reproductive strategies among different organisms.
Regulation of Germ Cell Development
Primitive germ cell markers play crucial roles in regulating gene expression patterns essential for germ cell development. Studying these markers helps in unraveling the intricate regulatory networks that govern germ cell specification, migration, and differentiation.
Potential Therapeutic Applications
Understanding primitive germ cell markers can have implications for regenerative medicine and fertility treatments. Insights gained from studying these markers may lead to the development of novel therapeutic strategies for reproductive disorders and infertility.
In summary, primitive germ cell markers are indispensable tools in developmental biology, providing essential information about germ cell development, pluripotency, differentiation, and reproductive health. Their study advances our understanding of fundamental biological processes and opens avenues for potential clinical applications in the field of reproductive medicine.
Case Study
Case 1: Malik HN, Singhal DK, Saini S, Malakar D. Derivation of oocyte-like cells from putative embryonic stem cells and parthenogenetically activated into blastocysts in goat. Sci Rep. 2020;10(1):10086.
Germ cells play a vital role in the perpetuation of animal species across generations. However, a concerning rise in infertility issues among livestock poses a significant threat to the economic progress of humanity. Addressing these challenges necessitates an alternative and robust strategy. Recent research has showcased that goat putative embryonic stem cells (ESCs) have been effectively differentiated into primordial germ cells and oocyte-like cells in vitro, leveraging bone morphogenetic protein-4 (BMP-4) and trans-retinoic acid (RA). These oocyte-like cells, characterized by a distinct zonapellucida, attracted neighboring somatic cells in the differentiating environment, forming cumulus-oocyte complexes (COCs). The putative COCs demonstrated expression of zonapellucida-specific (ZP1 and ZP2) and oocyte-specific markers. Furthermore, the presence of primordial germ cell-specific markers VASA, DAZL, STELLA, and PUM1 was confirmed at both protein and mRNA levels. Detailed visualization of the surface structure of these putative COCs was achieved through scanning electron microscopy. Subsequently, the putative COCs were parthenogenetically activated, leading to the development of healthy morula, blastocysts, and hatched blastocyst-like embryos. These findings have the potential to enhance the foundational understanding of mammalian germ cell biology and offer valuable clinical insights into infertility issues.
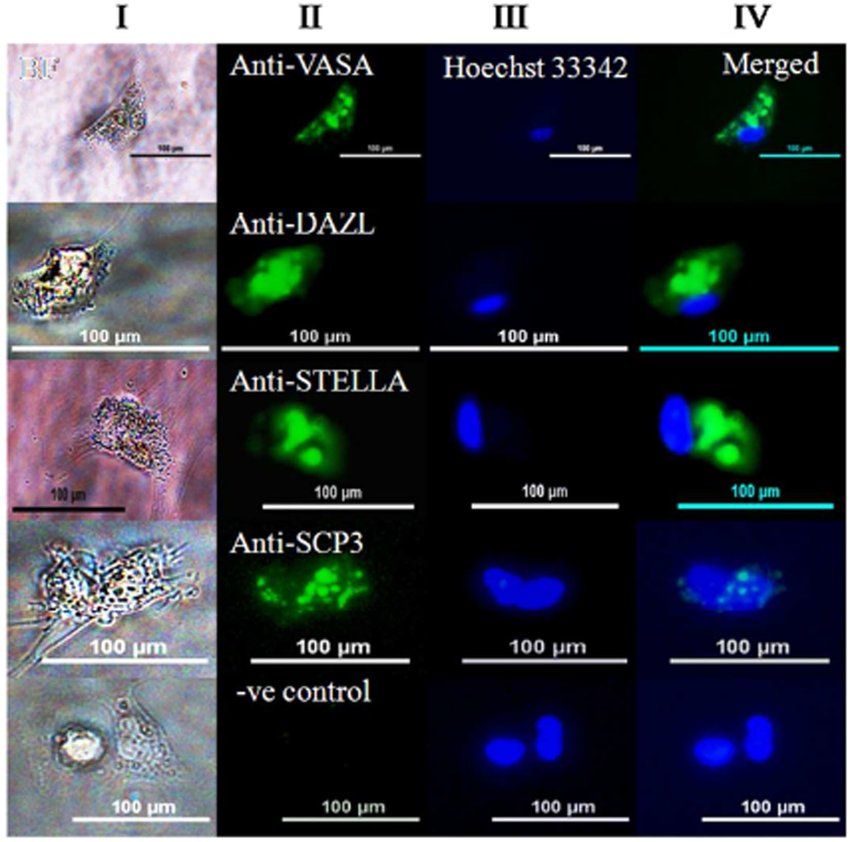 Fig.1 Detection of germ cell-specific markers in single PGC.
Fig.1 Detection of germ cell-specific markers in single PGC.Case 2: Sepulveda-Rincon LP, Wang YF, Whilding C, et al. Determining the potency of primordial germ cells by injection into early mouse embryos. Dev Cell. 2024;59(6):695-704.e5.
Primordial germ cells (PGCs) represent the earliest precursors of gametes in organisms. Under normal circumstances, PGCs typically differentiate into either oocytes or spermatozoa. Nonetheless, in vitro, PGCs can attain pluripotency by generating embryonic germ (EG) cells, and in vivo during teratocarcinogenesis. Traditional embryological investigations have directly evaluated the potential of PGCs through their introduction into pre-implantation embryos. As no discernible contribution to either embryos or adult mice was noted, PGCs have conventionally been deemed unipotent. In this study, it is illustrated that PGCs introduced into 8-cell embryos can initially survive, divide, and play a role in the formation of the developing inner cell mass. PGCs with inhibited apoptosis demonstrate enhanced survival within isolated epiblasts and have the capacity to generate naive pluripotent embryonic stem cell lines. However, their contribution to post-implantation embryos is restricted, with no functional integration observed. Conversely, PGC-like cells exhibit substantial involvement in mid-gestation chimeras. Consequently, it is suggested that PGC generation in vivo establishes a latent form of pluripotency that confines chimera contribution.
 Fig.2 Primordial germ cells can survive within the pre-implantation embryo and localize to the inner cell mass compartment.
Fig.2 Primordial germ cells can survive within the pre-implantation embryo and localize to the inner cell mass compartment.Case 3: Robinson M, Gilbert SF, Waters JA, Lujano-Olazaba O, Lara J, Alexander LJ, Green SE, Burkeen GA, Patrus O, Sarwar Z, et al. Characterization of SOX2, OCT4 and NANOG in ovarian cancer tumor-initiating cells. Cancers. 2021; 13(2):262.
The identification of tumor-initiating cells (TICs) traditionally depends on surface markers like CD133, CD44, CD117, and the aldehyde dehydrogenase (ALDH) enzyme, which shows varied expression levels among samples. A more dependable indicator of TICs might involve the presence of embryonic transcription factors that facilitate long-term self-renewal, multipotency, and dormancy. The hypothesis posits that SOX2, OCT4, and NANOG could be enriched in ovarian TICs, potentially signifying TICs with a heightened risk of relapse.
The study assessed a group of eight ovarian cancer cell lines cultivated in standard 2-D conditions versus spheroid-enriching 3-D environments, correlating expression patterns with growth behaviors, TIC marker presence, and resistance to chemotherapy. RNA-sequencing revealed elevated pathways associated with cell cycle regulation, particularly involving SOX2, in 3-D setups. High-grade serous ovarian cancer (HGSOC) lines exhibited extended doubling times, increased chemoresistance, and notably higher levels of SOX2, OCT4, and NANOG expression in 3-D settings. Cells marked by CD117+ or ALDH+/CD133+ displayed heightened SOX2, OCT4, and NANOG expression.
In vivo experiments employing limiting dilution implicated SOX2, rather than OCT4 or NANOG, in early tumor initiation. Patient data analysis suggested a more significant role for SOX2, in comparison to OCT4 or NANOG, in predicting tumor relapse potential. The collective results propose that SOX2 could serve as a more consistent marker for ovarian TICs involved in tumor repopulation post-chemotherapy. Future investigations focusing on SOX2 in TIC biology are poised to enhance comprehension of the underlying mechanisms driving ovarian cancer relapse.
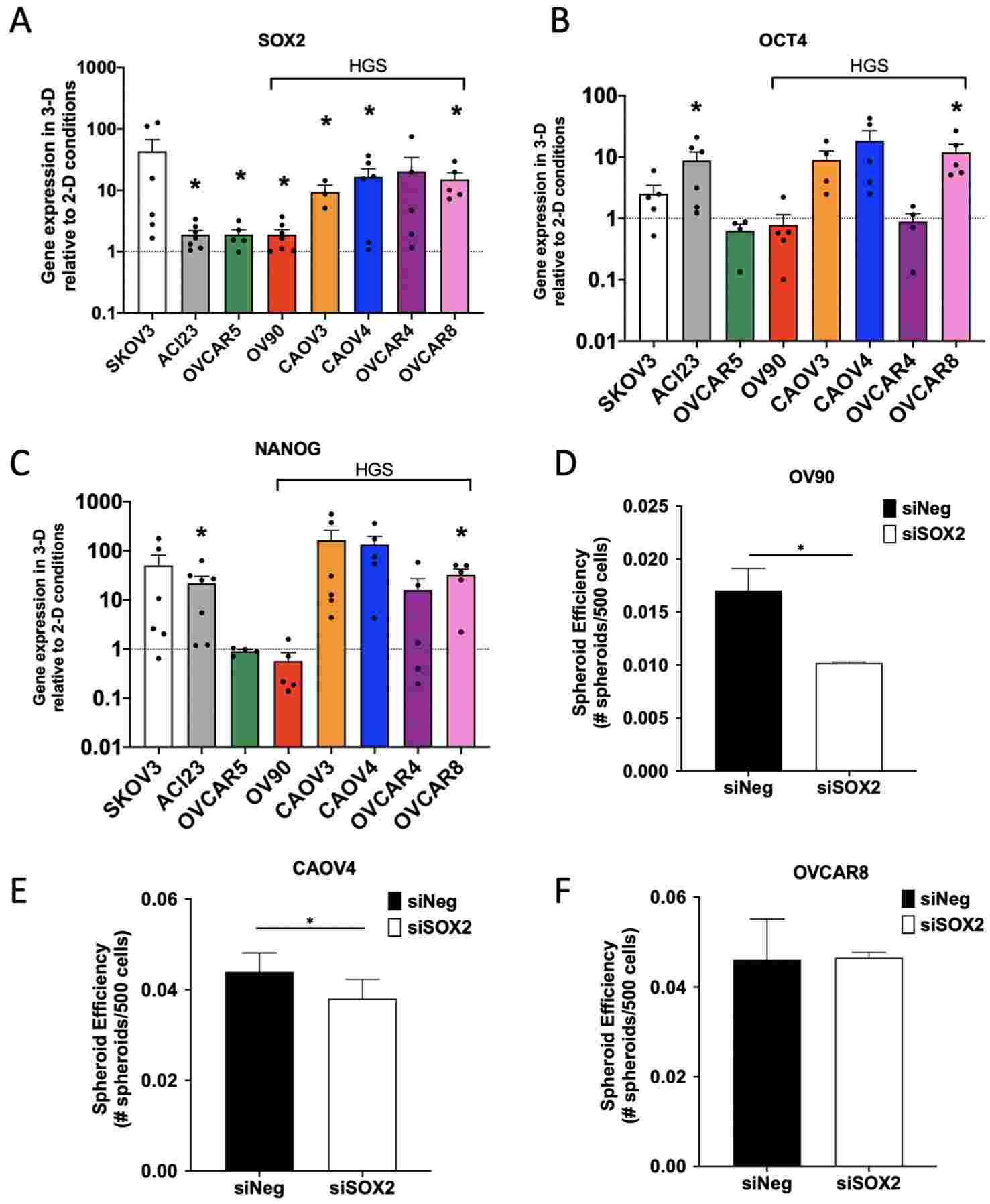 Fig.3 Enrichment of SOX2 is most consistent across ovarian cancer cell lines and contributes to spheroid formation efficiency.
Fig.3 Enrichment of SOX2 is most consistent across ovarian cancer cell lines and contributes to spheroid formation efficiency.Related References
- Raz E. Primordial germ-cell development: the zebrafish perspective. Nat Rev Genet. 2003;4(9):690-700.
- Hernández-Ochoa, Isabel & Paulose, Tinu & Flaws, J.A.. (2016). Ovarian Toxicology. 10.1016/B978-0-12-801238-3.10926-2.
- Ishii T. Human iPS cell-derived germ cells: current status and clinical potential. J Clin Med. 2014;3(4):1064-1083.
