Motor Neuronal Markers
Related Symbol Search List
Immunology Background
Background
Motor neurons are a type of neuron that is responsible for sending signals from the central nervous system to muscles or glands in the body. Motor neurons are an essential part of the motor system, as they help control movement and allow for voluntary muscle control. Damage to motor neurons can result in muscle weakness, paralysis, or other movement disorders.
Motor neuronal markers are crucial tools in neuroscience for identifying, characterizing, and studying motor neurons—the specialized nerve cells responsible for controlling voluntary muscle movements in the body. These markers play a pivotal role in distinguishing motor neurons from other cell types in the nervous system, aiding in the understanding of motor neuron development, function, and pathology.
By utilizing motor neuronal markers, researchers can track the development and degeneration of motor neurons in various disease models, such as amyotrophic lateral sclerosis (ALS) and spinal muscular atrophy (SMA). These markers also provide valuable insights into potential therapeutic targets for treating motor neuron-related diseases.
Importance of Motor Neuronal Markers
- Diagnostics: Identifying these markers can aid in the diagnosis of various neurodegenerative diseases.
- Research: Exploring the expression and function of these markers helps researchers understand motor neuron health and the mechanisms underlying neurodegeneration.
- Therapeutic Targets: Motor neuronal markers can serve as targets for therapeutic interventions aimed at protecting motor neurons or restoring function.
Common Motor Neuronal Markers
Here is a list of commonly used motor neuronal markers that are frequently employed to identify and study motor neurons in research:
| Markers | Details |
|---|---|
| Choline Acetyltransferase (ChAT) | ChAT is an enzyme involved in the synthesis of the neurotransmitter acetylcholine and is a well-established marker for cholinergic motor neurons. |
| Islet-1 (Isl1) | Isl1 is a transcription factor essential for the development of motor neurons and is commonly used as a marker for motor neuron progenitors and differentiated motor neurons. |
| Hb9 (Homeobox HB9) | Hb9 is a transcription factor crucial for the specification and differentiation of motor neurons in the spinal cord and is widely utilized as a marker for motor neurons. |
| Smi-32 (Non-Phosphorylated Neurofilament H) | Smi-32 is an antibody that targets non-phosphorylated neurofilament H, a cytoskeletal protein found in motor neurons, and is frequently used to label mature motor neurons. |
| NeuN (Fox-3 or RBFOX3) | NeuN is a neuronal nuclear antigen expressed in post-mitotic neurons, including motor neurons, and is commonly used as a marker for neuronal identity. |
| Peripherin | Peripherin is a type III intermediate filament protein expressed in subsets of motor neurons, particularly in peripheral motor neurons, and is used as a marker for specific motor neuron populations. |
| SOD1 (Superoxide Dismutase 1) | Mutations in the SOD1 gene are associated with familial ALS, making SOD1 a marker of interest in motor neuron disease research. |
| SMN (Survival Motor Neuron) | SMN protein is crucial for motor neuron survival, and its deficiency is linked to spinal muscular atrophy (SMA), making it a significant marker in SMA studies. |
| Pou3f1 (BRN3A) | Pou3f1 is a transcription factor involved in neuronal development and is used as a marker for specific subsets of motor neurons. |
| Neurofilament Proteins (NF-L, NF-M, NF-H) | Neurofilament proteins are major structural components of axons and are commonly used as markers for neuronal subtypes, including motor neurons. |
These markers collectively provide valuable tools for researchers to identify, characterize, and study motor neurons in both health and disease contexts, contributing to a better understanding of motor neuron biology and associated disorders.
Applications of Motor Neuronal Markers
Motor neuronal markers play a crucial role in both research and clinical settings, providing insights into motor neuron degeneration, regeneration, development, and function. Here's a discussion of these applications:
| Applications | Details | |
|---|---|---|
| Research Applications | Studying Neurodegeneration |
|
| Investigating Regeneration |
|
|
| Developmental Studies |
|
|
| Clinical Applications | Diagnostics |
|
| Monitoring Disease Progression |
|
|
| Personalized Medicine |
|
|
| Therapeutic Development |
|
|
Motor neuronal markers are vital tools in both research and clinical settings. They enhance our understanding of motor neuron biology, inform the development of diagnostic and therapeutic strategies, and pave the way for novel interventions aimed at combating motor neuron diseases. As research continues to unfold, these markers hold promise for improving patient outcomes and advancing the field of neurodegenerative disease treatment.
The Role of Motor Neuronal Markers in Diseases
Motor neuronal markers play a crucial role in the diagnosis and understanding of various neurological diseases, particularly those affecting motor neurons. Here are some key roles and examples of diseases associated with these markers:
| Characteristic | Disease | Markers | Role |
|---|---|---|---|
| Diagnostic Biomarkers | Amyotrophic Lateral Sclerosis (ALS) | SOD1, TDP-43, and FUS proteins. | Elevated levels of these proteins in neuron biopsies or cerebrospinal fluid (CSF) can indicate motor neuron degeneration typical of ALS. |
| Tissue Specificity | Spinal Muscular Atrophy (SMA) | SMN protein (Survival Motor Neuron) | Reduced levels of SMN in motor neurons contribute to SMA. Genetic testing for mutations in the SMN1 gene helps in diagnosing the disease. |
| Disease Progression | Parkinson's Disease | Alpha-synuclein aggregates in neurons | The presence of Lewy bodies, which contain alpha-synuclein, is used to understand the progression of motor symptoms in Parkinson's. |
| Predictive Value | Frontotemporal Dementia (FTD) | Progranulin and other tau proteins | Identifying changes in these markers can predict motor neuron involvement in FTD, helping in management and therapeutic approaches. |
| Therapeutic Targets | Clinical Trials | Neurofilament light chains (NfL) | High levels of NfL in blood or CSF reflect neurodegeneration and can be monitored to assess the efficacy of treatments in neurodegenerative diseases. |
| Understanding Pathophysiology | Multiple Sclerosis (MS) | Oligodendrocyte lineage markers | Alterations in these markers help in understanding demyelination and its impact on motor neurons in MS. |
| Potential for Regenerative Medicine | Motor Neuron Disease (MND) | Various stem cell markers are used in experimental therapies. | Understanding these markers aids in exploring potential regenerative therapies, such as stem cell transplantation targeting motor neuron repair. |
By studying these motor neuronal markers, researchers and clinicians can gain insights into disease mechanisms, improve diagnostic accuracy, and develop targeted therapies for motor neuron diseases.
Case Study
Case 1: Zhang QJ, Li JJ, Lin X, et al. Modeling the phenotype of spinal muscular atrophy by the direct conversion of human fibroblasts to motor neurons. Oncotarget. 2017;8(7):10945-10953.
In recent research, a study focused on spinal muscular atrophy (SMA), a severe autosomal recessive neurological disorder characterized by the targeted degeneration of spinal cord motor neurons. The study leveraged cellular reprogramming technology to generate patient-specific induced motor neurons from fibroblasts. By inducing the expression of 8 specific transcription factors, the fibroblasts transformed into dipolar structures with enlarging nuclei, eventually forming Tuj1-positive neurons by day 23.
Subsequent observations revealed induced neurons with multiple neurites expressing markers like Tuj1, HB9, ISL1, and CHAT after day 35. The conversion efficiencies were around 5.8% for the SMA group and 5.5% for the control group. Interestingly, SMA-induced neurons exhibited reduced neurite outgrowth rates compared to control neurons.
As the study progressed beyond day 60, the SMA-induced neurons displayed signs of neuronal degeneration, with noticeable fracturing of neurites. This work successfully established a feeder-free conversion system that generated SMA patient-specific induced motor neurons, offering a partial in vitro model of SMA pathology.
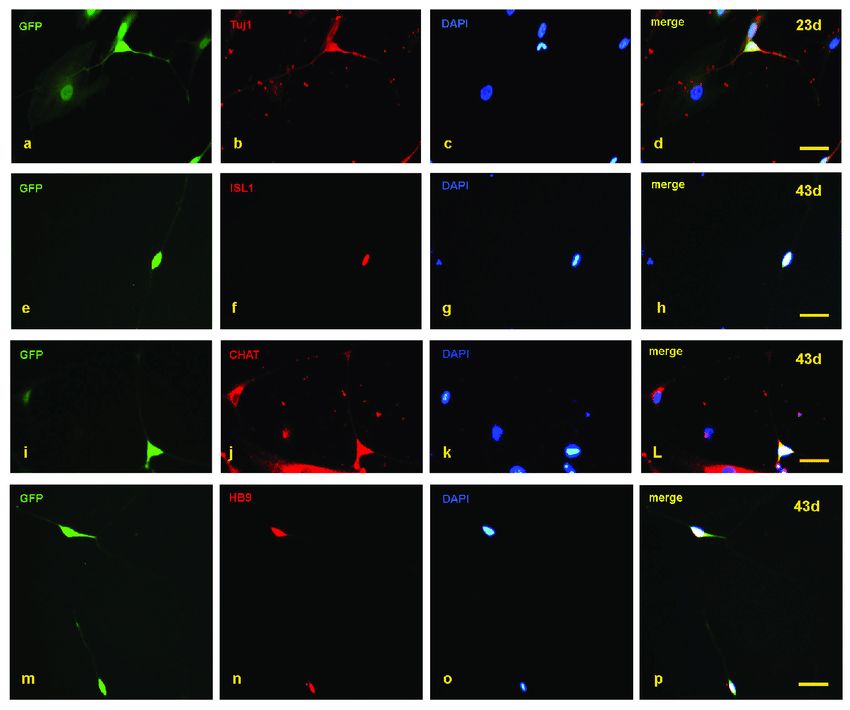 Fig.1 Detection of motor neuron markers with immunofluorescence staining.
Fig.1 Detection of motor neuron markers with immunofluorescence staining.Case 2: Chen S, Tian R, Luo D, Xiao Z, Li H, Lin D. Time-course changes and role of autophagy in primary spinal motor neurons subjected to oxygen-glucose deprivation: insights into autophagy changes in a cellular model of spinal cord ischemia. Front Cell Neurosci. 2020;14:38.
In the context of spinal cord ischemia, a severe clinical complication triggered by thoracoabdominal aortic surgery, severe trauma, or spinal column compression, spinal motor neurons (SMNs) are particularly vulnerable due to their high energy demands. While past studies have utilized animal models and organotypic tissue experiments to understand the pathogenesis and mechanisms of spinal cord ischemia, limited attention has been given to cellular models, mainly due to challenges in obtaining a sufficient number of pure primary SMNs for in vitro research.
Through optimization of SMN isolation and culture techniques, the research team has developed an enhanced primary SMN culture system, facilitating the creation of cellular models for mechanism studies. In their recent work, they set out to establish an in vitro model of spinal cord ischemia to observe the temporal changes in SMNs and explore the role of autophagy in these neurons during the ischemic process.
Their findings revealed that oxygen-glucose deprivation (OGD) led to the disruption of neural networks, and reduced cell viability in primary SMNs, with severity escalating as OGD duration increased. Interestingly, OGD treatment heightened autophagy activity, peaking at 5 hours. Subsequent investigations highlighted that inhibiting autophagy exacerbated cellular damage, underscoring the protective function of autophagy in safeguarding SMNs during the ischemic event.
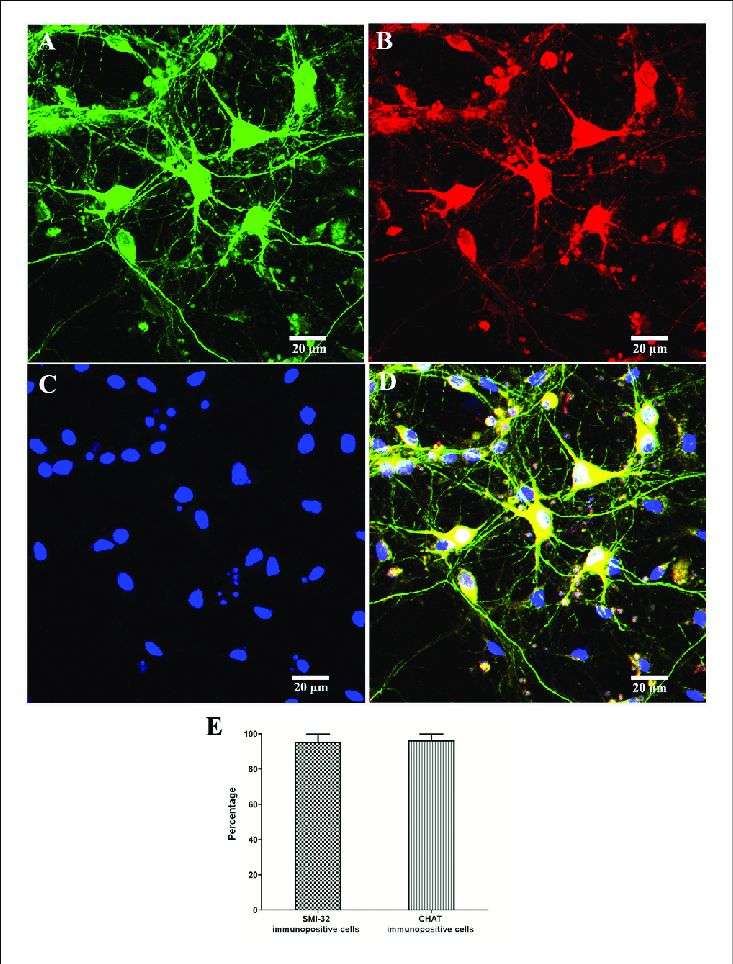 Fig.2 Identification and purity of SMNs by immunofluorescence with motor neuron-specific markers against SMI-32 and CHAT.
Fig.2 Identification and purity of SMNs by immunofluorescence with motor neuron-specific markers against SMI-32 and CHAT.Case 3: Schaller S, Buttigieg D, Alory A, et al. Novel combinatorial screening identifies neurotrophic factors for selective classes of motor neurons. Proc Natl Acad Sci U S A. 2017;114(12): E2486-E2493.
In their study, researchers investigated the combined effects of neurotrophic factors on the survival of developing motor neurons, a critical yet poorly understood area. They conducted screenings using 66 combinations of 12 neurotrophic factors on purified embryonic mouse motor neurons obtained through a specialized FACS technique, ensuring high viability and standardization.
Their findings revealed that hepatocyte growth factor (HGF), ciliary neurotrophic factor (CNTF), and Artemin exhibited potent and strictly additive survival effects by activating specific receptor complexes in distinct subsets of lumbar motor neurons. Specifically, HGF supported hindlimb motor neurons via c-Met, CNTF aided axial motor neurons through CNTFRα, and Artemin functioned as a crucial survival factor for parasympathetic preganglionic motor neurons through GFRα3/Syndecan-3 activation.
These results highlighted the selective promotion of survival in different classes of embryonic motor neurons by neurotrophic factors. The researchers suggested that similar investigations on postnatal motor neurons could lay the groundwork for utilizing a combination of neurotrophic factors therapeutically in degenerative motor neuron diseases like amyotrophic lateral sclerosis, spinal muscular atrophy, and spinobulbar muscular atrophy.
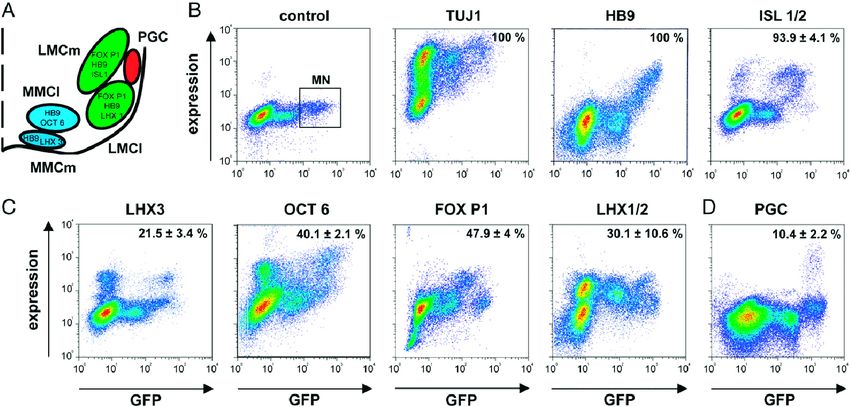 Fig.3 Quantitation of motor neuron subsets by double-color flow cytometry.
Fig.3 Quantitation of motor neuron subsets by double-color flow cytometry.Related References
- Katarina Stoklund Dittlau, Ludo Van Den Bosch, Why should we care about astrocytes in a motor neuron disease, Frontiers in Molecular Medicine, 3, (2023).
- Kaufmann P, Mitsumoto H. Amyotrophic lateral sclerosis: objective upper motor neuron markers. Current Neurology and Neuroscience Reports. 2002 Feb;2(1):55-60.
- Kanning KC, Kaplan A, Henderson CE. Motor neuron diversity in development and disease. Annual Review of Neuroscience. 2010 Jul 21;33(1):409-40.
