Cytokines and Chemokines in B Cell Development
Related Symbol Search List
Immunology Background
Background
Cytokines and chemokines play an important role in B cell development. Cytokines and chemokines play a crucial role in regulating the development and maturation of B cells, which are an important part of the immune system. These signaling molecules coordinate various stages of B cell development, including progenitor cell expansion, differentiation, survival, migration, and antibody production.
Cytokines are a class of secreted proteins that conduct signaling between cells and regulate cell function. In B cell development, cytokines can help regulate B cell proliferation, differentiation, and function.
Chemokines are a class of proteins that can induce specific cells to move toward the center of a chemical gradient. In B cell development, chemokines can guide naive B cells to migrate to destinations such as the bone marrow and lymphoid organs, thereby participating in the differentiation and maturation of B cells.
In general, cytokines and chemokines provide essential regulation and guidance for the development and function of B cells, and are key regulators and signaling molecules. The interaction between cytokines, chemokines, and their receptors forms a complex network of signaling pathways that strictly regulates B cell development and ensures normal immune function. Dysregulation of these signaling pathways can lead to abnormal B cell development, impair immune responses, and contribute to the development of immune diseases, including immunodeficiency, autoimmune diseases, and lymphoid malignancies.
Understanding the role of cytokines and chemokines in B cell development provides insights into the molecular mechanisms of immune system development and function. It also opens up potential therapeutic avenues for modulating B cell responses and treating immune-related diseases.
Key Cytokines and Chemokines in B Cell Development
| Molecules | Functions |
|---|---|
| CXCL12 (Chemokine C-X-C motif ligand 12) | CXCL12, also known as stromal cell-derived factor 1 (SDF-1), is a chemokine that plays a crucial role in B cell development. It is produced by stromal cells in the bone marrow and regulates the migration and localization of B cell progenitors. CXCL12 attracts B cell progenitors expressing its receptor, CXCR4, to specific niches within the bone marrow, creating a gradient that guides their migration and positioning. This chemokine is essential for the proper development and maturation of B cells. |
| CRLF2 (Cytokine Receptor-Like Factor 2) | CRLF2 is a cytokine receptor-like factor that is involved in B cell development. It forms a receptor complex with the interleukin-7 receptor alpha (IL7Rα) chain, leading to the activation of signaling pathways that are important for B cell development and survival. Dysregulation or genetic alterations in CRLF2 have been associated with certain types of B cell acute lymphoblastic leukemia (B-ALL). |
| Flt3l (FMS-like tyrosine kinase 3 ligand) | Flt3l is a cytokine that promotes the development of B cell progenitors and other immune cells. It acts through its receptor, Flt3, which is expressed on hematopoietic stem cells and B cell progenitors. Flt3l stimulates the expansion and differentiation of B cell precursors, contributing to the generation of mature B cells. |
| IL2RG (Interleukin-2 receptor gamma) | IL2RG is a shared component of receptors for several cytokines, including interleukin-2 (IL-2), IL-4, IL-7, IL-9, IL-15, and IL-21. Mutations in the IL2RG gene result in X-linked severe combined immunodeficiency (X-SCID), a condition characterized by the absence of functional B and T cells. IL2RG is required for the signaling of IL-7, which is crucial for B cell development and survival. |
| IL7 (Interleukin-7) | IL-7 is a key cytokine for B cell development. It is produced by stromal cells in the bone marrow and acts on B cell progenitors at multiple stages. IL-7 promotes the survival, proliferation, and differentiation of early B cell progenitors, facilitating their transition through the stages of B cell development. |
| IL7R (Interleukin-7 receptor) | IL-7R is the receptor for IL-7 and is expressed on B cell progenitors. It forms a complex with the cytokine receptor-like factor 2 (CRLF2) to activate signaling pathways that are important for B cell development and survival. Dysregulation or genetic alterations in IL7R have been associated with certain immune disorders and B cell malignancies. |
| TSLP (Thymic stromal lymphopoietin) | TSLP is a cytokine produced by epithelial cells in various tissues, including the thymus, lungs, and skin. It plays a role in B cell development by promoting the survival and proliferation of early B cell progenitors. TSLP signaling through its receptor, composed of the TSLP receptor (TSLPR) and IL-7Rα, influences B cell development and is involved in allergic and autoimmune diseases. |
These are just a few examples of the myriad cytokines and chemokines involved in B cell development. Their intricate interplay guides the different stages of B cell maturation, ensuring a diverse and functional B cell repertoire that contributes to immune responses against pathogens and the maintenance of immune homeostasis.
These cytokines and chemokines, along with their receptors, contribute to the regulation of B cell development, survival, proliferation, and migration. Dysregulation of these signaling pathways can lead to abnormalities in B cell development and function, contributing to immune disorders and malignancies. Understanding the roles of these mediators provides insights into the molecular mechanisms underlying B cell development and offers potential therapeutic targets for immune-related diseases. The immune system is a complex network, and the roles of cytokines and chemokines in B cell development continue to be explored and understood.
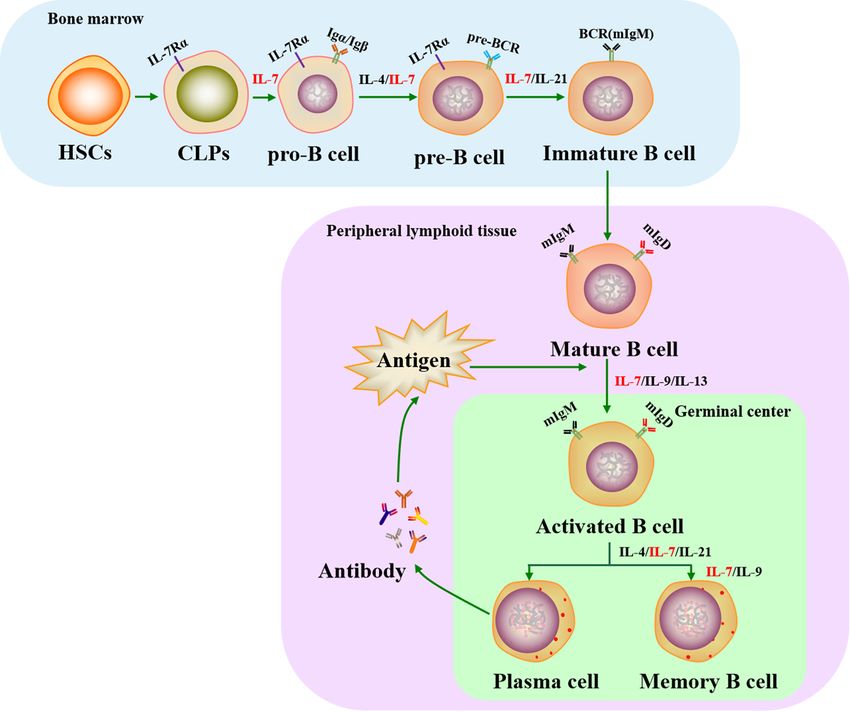 Fig.1 Role of IL-7 in the life cycle of B cells. (Slomp A, et al., 2018)
Fig.1 Role of IL-7 in the life cycle of B cells. (Slomp A, et al., 2018)Role of Cytokines and Chemokines in Abnormal B-Cell Development and Immune Diseases
Cytokines and chemokines play critical roles in regulating normal B cell development and maintaining immune homeostasis. However, dysregulation of these signaling molecules can lead to abnormal B cell development and contribute to the development of various immune diseases. Here are some examples of the role of cytokines and chemokines in abnormal B-cell development and immune diseases:
Cell malignancies
B-Aberrant cytokine and chemokine signaling can contribute to the development of B-cell malignancies, such as B-cell lymphomas and leukemias. Dysregulated production of cytokines like IL-10 and IL-6 can promote the survival and proliferation of malignant B cells. Additionally, chemokines like CXCL12 and CCL19, which normally guide B cell migration, can be involved in facilitating the infiltration and dissemination of malignant B cells.
Autoimmune diseases
In autoimmune diseases, the immune system mistakenly attacks the body's own tissues. Cytokines and chemokines can contribute to the dysregulation of B cells and the production of autoantibodies. For example, in systemic lupus erythematosus (SLE), dysregulated cytokines like IL-6 and IL-21 can promote B-cell hyperactivation and autoantibody production. Chemokines such as CXCL13 are implicated in the formation of ectopic lymphoid structures, called tertiary lymphoid organs, which contribute to the autoimmune response.
Immunodeficiencies
Defects in cytokine or chemokine signaling can lead to immunodeficiencies, where the immune system is compromised. For example, mutations in the IL2RG gene, encoding the common gamma chain shared by several cytokine receptors, can result in X-linked severe combined immunodeficiency (X-SCID). X-SCID patients have impaired B cell development and lack functional B and T cells. Similarly, deficiencies in other cytokines critical for B cell development, such as IL-7, can lead to immunodeficiency.
Allergic diseases
Allergic diseases, such as allergic rhinitis, asthma, and atopic dermatitis, involve dysregulated immune responses to harmless substances. Cytokines and chemokines play a crucial role in promoting B cell activation and immunoglobulin E (IgE) production, which are key features of allergic responses. Cytokines like IL-4 and IL-13 drive class switching to IgE, while chemokines such as thymic stromal lymphopoietin (TSLP) can enhance B cell survival and activation in allergic settings.
Chronic inflammation
Chronic inflammatory conditions, such as rheumatoid arthritis and inflammatory bowel disease, involve sustained immune activation and tissue damage. Cytokines, including tumor necrosis factor-alpha (TNF-α), IL-1β, and IL-6, contribute to chronic inflammation by promoting B cell activation, autoantibody production, and tissue destruction. Chemokines such as CXCL13 and CCL21 are involved in the recruitment of B cells and other immune cells to inflamed tissues.
Understanding the dysregulation of cytokines and chemokines in these conditions can provide insights into the underlying mechanisms of disease and potential therapeutic targets. Targeted therapies that modulate cytokine and chemokine signaling are being developed to treat immune diseases and restore normal B-cell development and function.
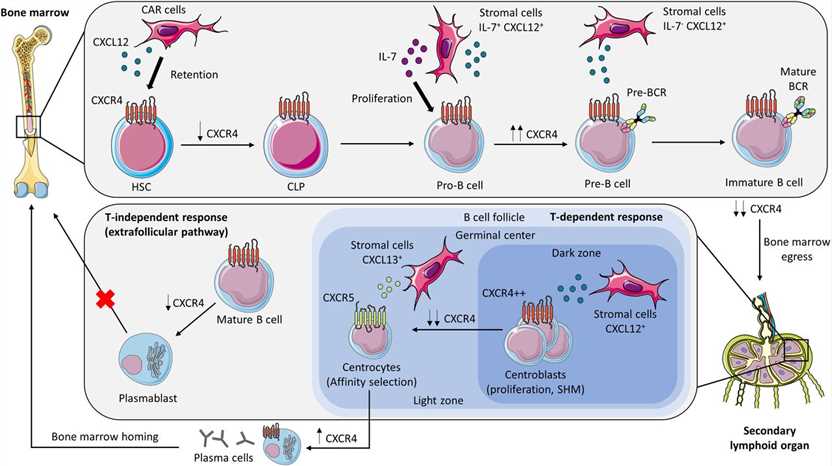 Fig.2 CXCR4 and B-cell development. (Giorgiutti S, et al., 2024)
Fig.2 CXCR4 and B-cell development. (Giorgiutti S, et al., 2024)Recent Research Progress and Potential Clinical Applications
Recent research has provided valuable insights into the roles of cytokines and chemokines in B cell development, leading to potential clinical applications. Here are some notable advancements and potential clinical implications:
Targeting cytokines for B cell malignancies
Cytokine signaling pathways have been identified as potential therapeutic targets for B cell malignancies. For example, inhibitors targeting IL-6 signaling, such as tocilizumab, have shown promise in inhibiting the growth and survival of B cell lymphomas. Similarly, monoclonal antibodies against IL-6, IL-10, and IL-21 are being explored as potential therapeutic agents for B cell malignancies.
Immunotherapies utilizing cytokines
Cytokines are being harnessed in immunotherapies to enhance B cell responses. For instance, IL-2 has been used in combination with monoclonal antibodies to improve the expansion and activation of B cells in the context of cancer immunotherapy. Additionally, IL-4 and IL-21 have been investigated as adjuvants to promote B cell-mediated immune responses in cancer vaccines.
Modulating chemokines for B cell trafficking
Chemokines and their receptors are potential targets for modulating B cell trafficking and migration. By manipulating chemokine signaling, it may be possible to enhance the recruitment of B cells to specific tissues or to inhibit their migration in autoimmune diseases or B cell malignancies. Such strategies could help regulate immune responses and improve therapeutic outcomes.
Cytokines in immunodeficiencies
Insights into the role of cytokines in immunodeficiencies, such as X-linked severe combined immunodeficiency (X-SCID), have paved the way for potential therapeutic interventions. Approaches like gene therapy or hematopoietic stem cell transplantation are being explored to restore the function of cytokine receptors and improve B cell development in these conditions.
Cytokines as biomarkers
Cytokines and chemokines are increasingly recognized as potential biomarkers for diagnosing and monitoring B cell-related diseases. Their levels and patterns of expression can provide valuable information about disease activity, response to therapy, and prognosis. Biomarker profiling of cytokines and chemokines may aid in personalized treatment strategies and monitoring therapeutic efficacy.
Combination therapies
Recent studies have highlighted the potential benefits of combining cytokines and chemokines with other therapeutic approaches. For example, combining cytokine-based immunotherapies with immune checkpoint inhibitors or targeted therapies has shown synergistic effects in B cell malignancies, improving treatment responses and patient outcomes.
It is worth noting that while these advancements hold promise, further research and clinical trials are necessary to validate their efficacy and safety in various B cell-related disorders. Nonetheless, the understanding of cytokines and chemokines in B cell development continues to provide a foundation for developing innovative therapeutic strategies and personalized treatment approaches in the field of immunology and oncology.
Case Study
Case 1: Rempenault C, Mielle J, Schreiber K, et al. CXCR5/CXCL13 pathway, a key driver for migration of regulatory B10 cells, is defective in patients with rheumatoid arthritis. Rheumatology (Oxford). 2022;61(5):2185-2196.
Chemokines (CKs) are key players of immune-cell homing and differentiation. CK receptors (CKRs) can be used to define T-cell functional subsets. The authors aimed to characterize the CKR profile of the regulatory B-cell subset B10+ cells and investigate the CKs involved in their migration and differentiation in healthy donors and patients with RA.
CXCR5 was expressed at a higher level on the B10+ cell surface as compared with other B cells (referred to as B10neg cells). In line with this, its ligand CXCL13 preferentially attracted B10+ cells over B10neg cells. Interestingly, synovial fluid from RA patients contained high levels of CXCL13 and induced strong and preferential migration of B10+ cells. Besides its role in attracting B10+ cells, CXCL13 also promoted IL-10 secretion by B cells. In RA patients, the level of CXCR5 on B-cell surface was reduced. The preferential migration of RA B10+ cells toward CXCL13-rich SF was lost and CXCL13 stimulation triggered less IL-10 secretion than in healthy donors.
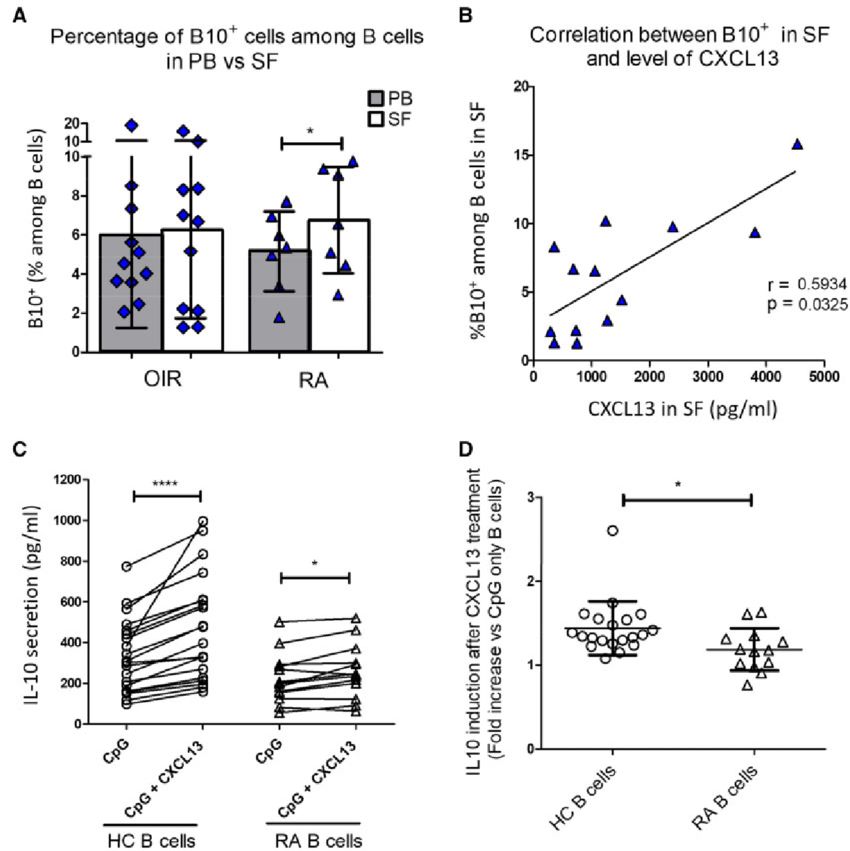 Fig.1 Local impact of CXCL13 on B cells (CD19+).
Fig.1 Local impact of CXCL13 on B cells (CD19+).Case 2: El-Kassar N, Flomerfelt FA, Choudhury B, et al. High levels of IL-7 cause dysregulation of thymocyte development. Int Immunol. 2012;24(10):661-671.
B cells developing in IL-7 Tg thymus did not arise from ETPs. The study investigated other progenitor populations and found an increased presence of B220-AA4.1+ and B220+AA4.1+ cells from IL-7 Tg LSK cells when co-cultured with DLL1-expressing stromal cells. These populations expressed IL-7R and corresponded to common lymphoid progenitors (CLPs) and CLP-2 cells, which have lymphoid-restricted BM-progenitors with higher B-cell potential. CLP-2 cells were observed in the thymi of IL-7 Tg mice but absent in WT mice, and they were also found in the BM of both IL-7 Tg and WT mice. CLP-2 progenitors from normal BM developed into B and T cells when cultured on OP9 and OP9-DL1, respectively. Additionally, high IL-7 levels inhibited thymocyte development in BM-progenitor and OP9-DL1 co-cultures but led to an increase in cells expressing B-cell markers AA4.1, B220, and CD19.
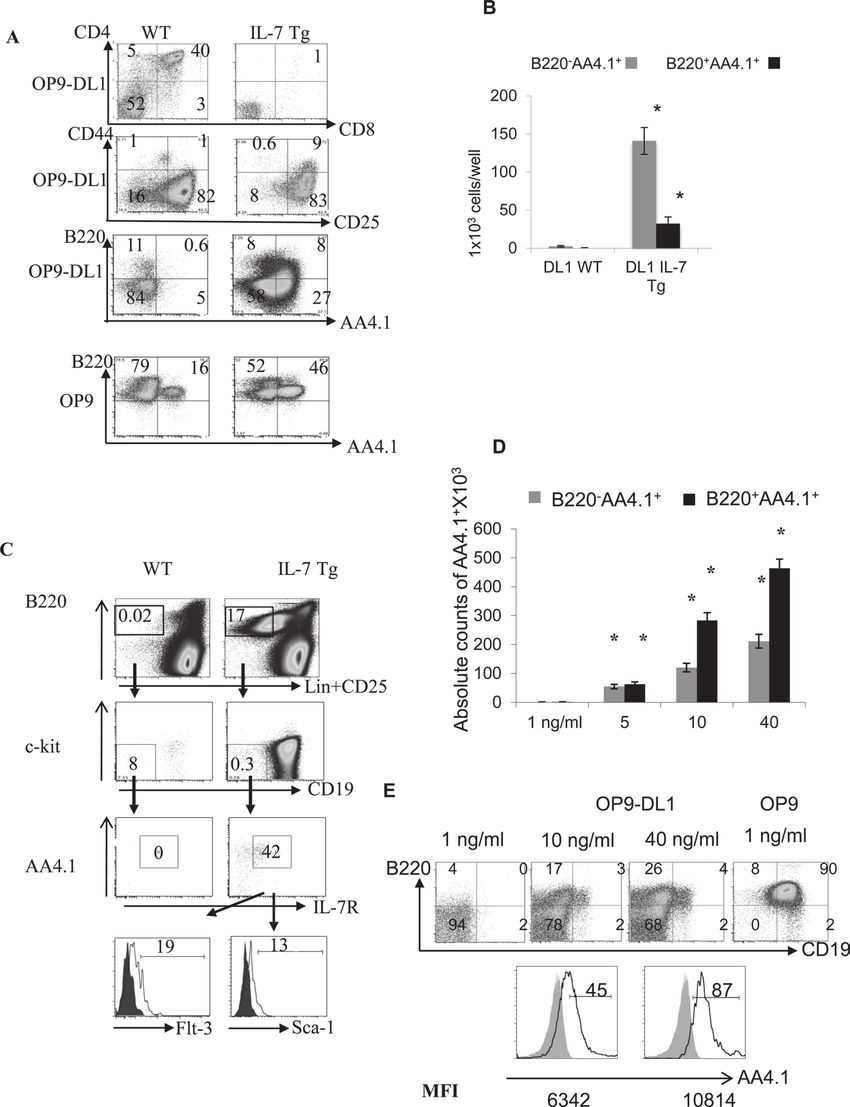 Fig.2 Increase of cells with B-cell markers instead of DP cells on OP9-DL1 stromal cells in presence of high IL-7.
Fig.2 Increase of cells with B-cell markers instead of DP cells on OP9-DL1 stromal cells in presence of high IL-7.References
- Acosta-Rodríguez EV, Merino MC, Montes CL, Motrán CC, Gruppi A. Cytokines and chemokines shaping the B-cell compartment. Cytokine Growth Factor Rev. 2007;18(1-2):73-83.
- Giorgiutti S, Rottura J, Korganow AS, Gies V. CXCR4: from B-cell development to B cell-mediated diseases. Life Sci Alliance. 2024;7(6):e202302465.
