IVD of Phleum pratense Allergy
🧪 Phlp5b-1334P
Source: E.coli
Species: Phleum pratense
Tag: His&SUMO
Conjugation:
Protein Length: 20-284 a.a.
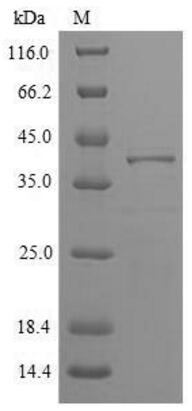
🧪 PHLPI-1648P
Source: E.coli
Species: Phleum pratense
Tag: His
Conjugation:
Protein Length: IIe24-Lys263

🧪 PHLPII-1649P
Source: E.coli
Species: Phleum pratense
Tag: His
Conjugation:
Protein Length: Val27-Glu122

Phleum pratense
Phleum pratense is a common lawn grass whose pollen is the main allergen confirmed to cause grass fever. Phleum pratense pollen allergen is a plant-based allergen that mainly enters the human body through pollen particles in the air, thereby stimulating the body's immune system to produce allergic reactions, such as dermatitis, anaphylactic shock, asthma, and other symptoms. Current research shows that Phleum pratense pollen allergens mainly include Phl p 1, Phl p 2, Phl p 5, Phl p 7, and Phl p 12. In vitro diagnosis (IVD) of grass pollen allergy is usually based on the detection of IgE antibodies in serum.

Main Steps of IVD for Phleum pratense Allergy
- Immunological Test
Detection of IgE levels in serum, including total IgE determination and specific IgE determination. These assays can detect a patient's specific immune response to Phleum pratense pollen.
- Skin Prick and Intradermal Test
In these tests, pollen extract is pierced or injected into the skin and the skin's reaction is observed to determine whether an allergic reaction occurs.
- Biosensor Technology
This technology can detect the electrical signal when pollen allergens combine with IgE antibodies, thereby quickly and accurately detecting whether the patient is allergic to pollen.
Creative BioMart provides high-quality recombinant Phleum pratense allergen proteins used for IVD, including ELISA, lateral flow assay, western blot, and other immunoassays.
Highlights of Our Products
- Higher safety and repeatability.
- High purity for compliance with experiment standards.
- High specificity. The molecular structure and biological activity of recombinant allergens are similar to natural allergens and can effectively stimulate immune responses in allergic patients.
- Easy to scale up production. A large number of specific proteins can be obtained through process amplification, without being limited by the content of natural extracts and materials.
Our Outstanding Advantages
- Provide a series of recombinant allergen proteins for IVD applications, including food, pollen, and mite allergens.
- Strong technical team, advanced scientific research equipment, and technology that can provide high-quality services.
- A rich variety of IVD products to meet the different needs of customers and provide customers with comprehensive scientific research support.
- Ensure high standards of service quality and delivery of timely and accurate IVD services to customers, with a focus on providing the best possible scientific research experience.
Clinical Related Information
Cause of Phleum pratense Allergy
Phleum pratense allergy is caused by the immune system reacting to the proteins (allergens) found in the pollen of Timothy grass. When an allergic individual inhales the pollen, their immune system mistakes it for a harmful substance and triggers an allergic response.
Symptoms of Phleum pratense Allergy
- Sneezing
- Runny or congested nose (allergic rhinitis)
- Itchy or watery eyes (allergic conjunctivitis)
- Coughing
- Wheezing or shortness of breath
- Asthma exacerbation
- Itchy throat or ears
Case Study
Case 1: Sola Martínez FJ, Barranco Jiménez RM, Martín García C, Senent Sánchez C, Blanco Guerra C, Fernández-Rivas M, Vega Castro A, Dávila González I, Carbonell Martínez A, Panizo Bravo C, Gómez Torrijos E, Rodríguez Gil D, Palacios Peláez R. Intradermal Phleum pratense allergoid immunotherapy. Double-blind, randomized, placebo-controlled trial. Clin Exp Allergy. 2020 Dec;50(12):1352-1361. doi: 10.1111/cea.13740. Epub 2020 Oct 25. PMID: 32946612; PMCID: PMC7756767.
The objective of this article is to evaluate the efficacy and safety of immunotherapy with an allergen extract of glutaraldehyde-polymerized Phleum pratense, administered intradermally, in patients with rhinoconjunctivitis sensitized to grass pollen.
 Fig2. Specific IgE against Phleum pratense, Phl p 1 and Phl p 5 levels during immunotherapy. *P < .05
Fig2. Specific IgE against Phleum pratense, Phl p 1 and Phl p 5 levels during immunotherapy. *P < .05Case 2: Xu Y, Guan K, Sha L, Zhang J, Niu Y, Yin J, Wang L. Sensitization Profiles of Timothy Grass Pollen in Northern China. J Asthma Allergy. 2021 Nov 30;14:1431-1439. doi: 10.2147/JAA.S334183. PMID: 34876820; PMCID: PMC8643203.
Grass pollen is an important cause of IgE-mediated allergy in countries worldwide, especially within Europe. However, there has been no research on grass pollen allergy in northern China. The authors aimed to determine the status of grass pollen allergy and the sensitization patterns to Phleum pratense (P. pratense) in northern China.
 Fig3. Pollen concentration in three areas. Pollen monitoring was performed by a gravitational method using a modified Durham pollen sampler described by Ye.
Fig3. Pollen concentration in three areas. Pollen monitoring was performed by a gravitational method using a modified Durham pollen sampler described by Ye.Case 3: Neuper T, Neureiter D, Sarajlic M, Strandt H, Bauer R, Schwarz H, Suchanek P, Korotchenko E, Dillon SR, Hammerl P, Stoecklinger A, Weiss R, Horejs-Hoeck J. IL-31 transgenic mice show reduced allergen-induced lung inflammation. Eur J Immunol. 2021 Jan;51(1):191-196. doi: 10.1002/eji.202048547. Epub 2020 Jul 21. PMID: 32648940; PMCID: PMC7818168.
The main aim of this study was to unravel the contribution of IL-31 to allergen-induced lung inflammation. We analyzed lung inflammation in response to the timothy grass (Phleum pratense) pollen allergen Phl p 5 in C57BL/6 wild-type (wt) mice, IL-31 transgenic (IL-31tg) mice, and IL-31 receptor alpha-deficient animals (IL-31RA-/- ).
 Fig4. Analysis of histopathology and cellular infiltration in wt, IL‐31tg, and IL‐31RA−/− mice after mock or Phl p 5 challenge. Perfused lungs of Phl p 5‐challenged mice were stained with (A) H&E to assess histomorphology (scale bar, 100 μm), (B) Giemsa to identify infiltrating lymphocytes and eosinophils (scale bar, 50 μm), (C) PAS to determine mucus production (scale bar, 50 μm), or (D) trichrome staining to monitor epithelial thickness (scale bar, 50 μm).
Fig4. Analysis of histopathology and cellular infiltration in wt, IL‐31tg, and IL‐31RA−/− mice after mock or Phl p 5 challenge. Perfused lungs of Phl p 5‐challenged mice were stained with (A) H&E to assess histomorphology (scale bar, 100 μm), (B) Giemsa to identify infiltrating lymphocytes and eosinophils (scale bar, 50 μm), (C) PAS to determine mucus production (scale bar, 50 μm), or (D) trichrome staining to monitor epithelial thickness (scale bar, 50 μm).











