Bacterial Expression Systems (E. coli / Bacillus)
Our bacterial protein expression services leverage cutting-edge microbial platforms, including E. coli expression systems and Bacillus subtilis hosts, to deliver customized solutions for recombinant protein production. From codon-optimized gene synthesis and plasmid vector design to large-scale protein expression, purification (affinity/SEC/IEX chromatography), and solubility optimization through refolding protocols, we ensure end-to-end project support for pharmaceutical development, industrial biotechnology, and academic research applications. Our optimized protocols achieve >90% purity levels with industry-leading yields, backed by quality control through SDS-PAGE, Western blotting, and mass spectrometry analysis. As for fermentation scale, we have from 1 to 20,000 liters of bioreactors available to meet your requirements. Streamline your therapeutic protein production, enzyme engineering, or structural biology projects with our GMP-compliant, scalable microbial expression solutions. Contact our protein experts today for tailored expression strain selection and process development strategies.
Background
What are Bacterial Expression Systems?
Bacterial expression systems have been proved to be the preferred option for laboratory investigations and initial development in commercial manufacture in comparison with various other expression systems. The advantages of bacterial expression include thorough undersanding of the system, low cost, high expression yield, ease of scale-up, and short turnaround time.
In the latest decade, gram-positive Bacilli strains have become very popular hosts for industrial and pharmaceutical protein production. Many pharmaceutically relevant proteins were successfully expressed in Bacilli strains. In contrast to the well-known E.coli , Bacilli strains have the general advantage that its outer membrane has no LPS. Furthermore, the Bacilli strains are attractive hosts because of their natural secretion capacity, which can export proteins directly into the extracellular medium.
Creative BioMart's Bacillus subtilis system is an ideal option for the expression of monomeric protein products at high expression levels, combined with our proprietary fermentation protocols allow for fast process development and scale-up to support early clinical testing.

Advantages of Bacterial Expression Systems
Simple, well-understood genetics
Easy to manipulate
Fast expression with short doubling time
Easy to purify from inclusion bodies
Well-established labeling protocols for stability studies
Established regulatory track record
Easy to scale up by fermentation
Minimal culturing cost
Service Details
Custom Solutions for Diverse Applications
Tailor every step to your project’s needs:
- Therapeutic proteins : GMP-grade expression for vaccines, cytokines, and antibody fragments.
- Structural biology : Isotope-labeled proteins (15N/13C) for NMR/X-ray crystallography.
- Protein engineering : High-throughput screening for industrial biocatalysts.
Turnkey Services: From Gene to Protein
Streamline R&D timelines with our end-to-end turnkey services , integrating gene synthesis, vector construction, fermentation optimization, and downstream processing. Ideal for startups and enterprises seeking scalable, cost-effective alternatives to mammalian expression systems.
1
Gene Synthesis Plus Codon Optimization
- Leading codon optimization system
- Analysis and optimization of rare codons, codon bias and other factors
Timeline: 1-2 weeks
2
Vector Construction
- Possess dozens of expression vectors
- Clone target gene into expression vector
- Plasmid sequencing
- Plasmid preparation
Timeline: 1 week
3
Protein Expression and Purification
- Small-scale fermentation culture
- Expression condition optimization
- Protein expression evaluation
- Various protein purification methods
Timeline: 1-2 weeks
4
Optional Services
- Protein refolding
- Secondary purification
- Tag removal
- Endotoxin removal
- Protein labeling
- Activity assay
- Protein analysis
Timeline: 1-2 weeks
5
Large Scale/ Fermentation (1L - 5,000L)
- Standard fermentation
- High cell density fermentation
Timeline: 1-8 weeks
6
Quality Control
- SDS-PAGE
- Western blot
- Bradford assay
- Mass spectrometry
Timeline: <1 week
Sample Submission Requirements
Essential Requirements
- Target Gene Sequence : Please provide the DNA or protein sequence of your target gene. This is crucial for accurate gene synthesis and vector construction.
- Sample Quantity : If submitting DNA samples (plasmid or PCR product), a minimum of 3 µg is required to ensure sufficient material for processing.
- Documentation : Include any relevant sequence files (e.g., FASTA format) or vector maps if applicable. This helps us understand your specific needs better.
Optional but Helpful Information
- Preferred Host Strain : Let us know if you have a preference for the bacterial host. We can also recommend the most suitable host based on your goals.
- Expression Conditions : If you have specific induction temperatures, IPTG concentrations, or other expression preferences, please share them. Otherwise, we will optimize these conditions for you.
- Purification and Application Details : Inform us about your desired protein purity level and intended downstream applications (e.g., structural studies, functional assays). This helps us tailor the purification process to meet your needs.
Deliverables
- Purified protein (supplied in liquid or lyophilized form)
- Gene sequencing report
- QC report
- SDS-PAGE/ HPLC/ SEC
- Activity data if applicable
Our extensive experience and flexible strategies enable Creative BioMart to provide high quality proteins in scales and purity levels tailored to your research.
Why Choose Us?
- PhD-Driven Expertise & Dedicated Support. Our team of experienced PhD-level scientists specializes in microbial expression challenges, delivering end-to-end project guidance from construct design to results transfer. Benefit from 15+ years of collective expertise in recombinant protein production for applications spanning biotherapeutics to enzyme engineering.
- Proprietary Fermentation & Refolding Methodologies. Leverage our advanced high-density fermentation processes developed for E. coli and Bacillus systems, designed to enhance biomass yield while minimizing metabolic stress. For insoluble proteins, our optimized refolding platform integrates stepwise dialysis, redox buffer systems, and AI-assisted additive screening to recover functional conformations efficiently.
- High Success Rates Across Diverse Applications. With a 98% gene-to-protein success rate for soluble targets, we’ve supported 500+ global clients in academia, pharma, and biotech. Our iterative optimization approach ensures robust expression and purification outcomes, backed by transparent progress reporting.
- Global Reach, Localized Customer Support. Leverage 24/7 project tracking via our secure portal and regional hubs in North America, Europe, and Asia. Whether you’re a startup seeking turnkey solutions or an enterprise requiring GMP-compliant processes, our tailored support adapts to your time zone and compliance needs.
- Trusted by Innovators Worldwide. Join 200+ satisfied clients who praise our transparent communication and problem-solving agility. Access verified testimonials highlighting accelerated IND-enabling studies, cost savings vs. mammalian systems, and seamless scalability from mg to kg batches.
Case Study
* NOTE: We prioritize confidentiality in our services to safeguard technology and intellectual property for enhanced future value and protection. The following case study has been shared with the client's consent.
Project Name: Custom Protein Production in E. coli
Goal
The project aims to construct five variant expression strains, optimize expression conditions, and purify the corresponding target proteins. The customer provided sequence information for the five variants. The workflow includes gene synthesis, vector construction, trial expression, purification, and refolding processes.
Results
Expression and Purification:
- Construct 1-5 were successfully expressed as inclusion bodies in Rosetta (DE3) and BL21 (DE3) strains.
- The expression levels of constructs 2-5 were relatively high, with levels increasing at higher induction temperatures (30°C and 37°C). However, protein impurities were observed in the lysates at these temperatures.
- Construct 1 showed lower expression levels compared to the other constructs, regardless of the host strain or induction conditions.
- The purification and refolding processes resulted in low final concentrations and purities of the refolded proteins. Further optimization is required to improve these processes.
Refolding and Stability:
- Refolding trials using dialysis and in-column methods showed that the target proteins could be refolded, but the final concentration and purity were generally low.
- Stability tests indicated that Tris buffer pH 8.5 + 10% glycerol was the most suitable buffer for maintaining protein stability. This buffer can be used as the final buffer for the target proteins.
Conclusions
- All constructs were successfully expressed as inclusion bodies, but the expression levels varied significantly, with construct 1 showing the lowest expression.
- The refolding process needs further optimization to improve protein concentration and purity.
- The stability of the refolded proteins was best maintained in Tris buffer pH 8.5 + 10% glycerol .
-
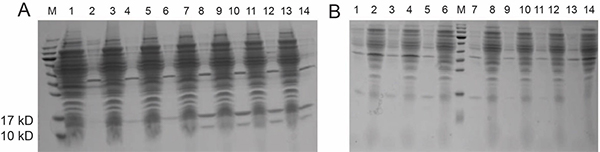 Fig. 1. SDS-PAGE analysis of construct 1-BL21 protein expression.
Fig. 1. SDS-PAGE analysis of construct 1-BL21 protein expression.A. lysis buffer (pH 8.5); B. lysis buffer (pH 4.5).
“neg“ denotes negative control; “0.5/1.0“ denotes the final concentration of IPTG; “S/P” denotes supernatant and precipitate, respectively.
Lane1: 1-BL21-neg-S Lane2: 1-BL21-neg-P
Lane3: 1-BL21-18°C -0.5-S Lane4: 1-BL21-18°C -0.5-P
Lane5: 1-BL21-18°C -1.0-S Lane6: 1-BL21-18°C -1.0-P
Lane7: 1-BL21-30°C -0.5-S Lane8: 1-BL21-30°C -0.5-P
Lane9: 1-BL21-30°C -1.0-S Lane10: 1-BL21-30°C -1.0-P
Lane11: 1-BL21-37°C -0.5-S Lane12: 1-BL21-37°C -0.5-P
Lane13: 1-BL21-37°C -1.0-S Lane14: 1-BL21-37°C -1.0-P -
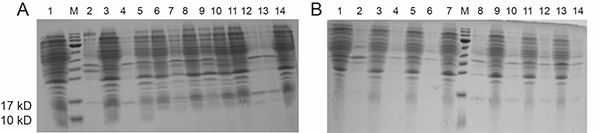 Fig. 2. SDS-PAGE analysis of construct 1-Rosetta protein expression.
Fig. 2. SDS-PAGE analysis of construct 1-Rosetta protein expression.A. lysis buffer (pH 8.5); B. lysis buffer (pH 4.5).
“neg“ denotes negative control; “0.5/1.0“ denotes the final concentration of IPTG; “S/P” denotes supernatant and precipitate, respectively.
Lane1: 1-Rosetta-Neg-S Lane2: 1-Rosetta-Neg-P
Lane3: 1-Rosetta-18°C -0.5-S Lane4: 1-Rosetta-18°C -0.5-P
Lane5: 1-Rosetta-18°C -1.0-S Lane6: 1-Rosetta-18°C -1.0-P
Lane7: 1-Rosetta-30°C -0.5-S Lane8: 1-Rosetta-30°C -0.5-P
Lane9: 1-Rosetta-30°C -1.0-S Lane10: 1-Rosetta-30°C -1.0-P
Lane11: 1-Rosetta-37°C -0.5-S Lane12: 1-Rosetta-37°C -0.5-P
Lane13: 1-Rosetta-37°C -1.0-S Lane14: 1-Rosetta-37°C -1.0-P -
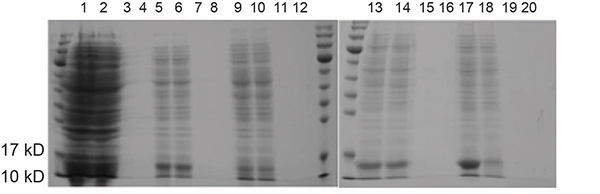 Fig. 3. SDS-PAGE analysis of purification of inclusion body proteins.
Fig. 3. SDS-PAGE analysis of purification of inclusion body proteins.“1-5-Rosetta“ denotes construct 1-5 Rosetta expression protein; “P, F and E“ denotes the pre-column fractions, Flow-through fractions and elution fractions, respectively.
Lane1: 1-Rosetta-P Lane2: 1-Rosetta-F Lane3-4: 1-Rosetta-E
Lane5: 2-Rosetta- P Lane6: 2-Rosetta-F Lane7-8: 2-Rosetta-E
Lane9: 3-Rosetta-P Lane10: 3-Rosetta-F Lane11-12: 3-Rosetta-E
Lane13: 4-Rosetta-P Lane14: 4-Rosetta-F Lane15-16: 4-Rosetta-E
Lane17: 5-Rosetta-P Lane18: 5-Rosetta-F Lane19-20: 5-Rosetta-E -
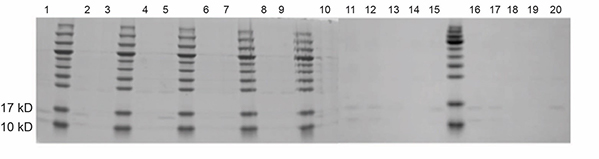 Fig. 4. SDS-PAGE analysis of refolded protein dialyzed by different refolding buffers.
Fig. 4. SDS-PAGE analysis of refolded protein dialyzed by different refolding buffers.“1-5-Rosetta“ denotes construct 1-5 Rosetta expression protein
Lane1: 1-Rosetta-Tris+glycerol Lane2: 2-Rosetta-Tris+glycerol
Lane3: 3-Rosetta-Tris+glycerol
Lane4: 4-Rosetta-Tris+glycerol
Lane5: 5-Rosetta-Tris+glycerol
Lane6: 1-Rosetta-PBS+glycerol
Lane7: 2-Rosetta-PBS+glycerol
Lane8: 3-Rosetta-PBS+glycerol
Lane9: 4-Rosetta-PBS+glycerol
Lane10: 5-Rosetta-PBS+glycerol
Lane11: 1-Rosetta-Tris
Lane12: 2-Rosetta-Tris
Lane13: 3-Rosetta-Tris
Lane14: 4-Rosetta-Tris
Lane15: 5-Rosetta-Tris
Lane16: 1-Rosetta-PBS
Lane17: 2-Rosetta-PBS
Lane18: 3-Rosetta-PBS
Lane19: 4-Rosetta-PBS
Lane20: 5-Rosetta-PBS
-
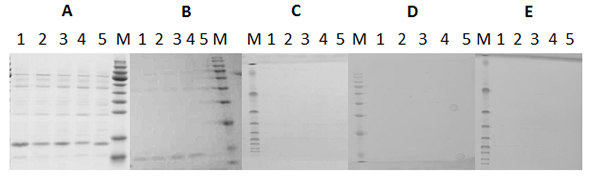 Fig. 5. SDS-PAGE analysis of protein stability in PBS buffer, pH 8.5.
Fig. 5. SDS-PAGE analysis of protein stability in PBS buffer, pH 8.5.Lane M: Marker; Lane 1: Construct 1; Lane 2: Construct 2; Lane 3: Construct 3; Lane 4: Construct 4; Lane 5: Construct 5.
-
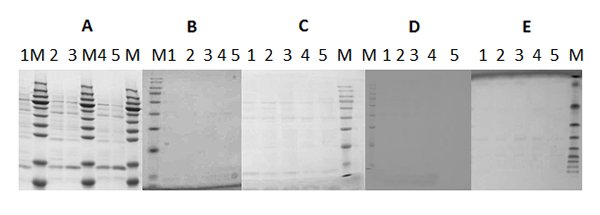 Fig. 6. SDS-PAGE analysis of protein stability in PBS buffer+10% glycerol, pH 8.5.
Fig. 6. SDS-PAGE analysis of protein stability in PBS buffer+10% glycerol, pH 8.5.Lane M: Marker; Lane 1: Construct 1; Lane 2: Construct 2; Lane 3: Construct 3; Lane 4: Construct 4; Lane 5: Construct 5.
Table 1 Final proteins from 5 constructs delivered to customer; all expression conditions identical except the plasmid.
| Construct (only variable) | Host cell | Induction by IPTG | Induction temperatures | Protein production in supernatant or inclusion bodies | Protein refolding | pH | Final buffer |
|---|---|---|---|---|---|---|---|
|
Construct 1 |
Rosetta |
0.5 mM |
37 °C |
Inclusion bodies |
Dialysis refolding with guanidinium chloride |
8.5 |
Tris buffer pH 8.5 +10% glycerol |
|
Construct 2 |
... |
... |
... |
... |
... |
... |
... |
Customer Testimonials
-
"Creative BioMart’s codon optimization for E. coli expression slashed our antigen production timeline by 30%. Their troubleshooting support for vector construction issues was invaluable during critical IND-enabling studies."
-Dr. Clara Bennett, Senior Scientist | Vaccine Development Lab
-
"We struggled with insoluble lipases until their protein refolding system achieved 85% active recovery. The seamless transition from small-scale protein purification to pilot fermentation saved us months of R&D."
-Prof. Raj Patel, Head of Protein Engineering | Industrial Enzyme Manufacturer
-
"Outsourcing our microbial fermentation scale-up reduced costs by 40% vs. in-house efforts. Their proprietary high-density protocols and real-time pH/O2 monitoring ensured batch consistency for antibody fragment production."
-Dr. Anika Vogt, R&D Head | Biopharmaceutical Firm
-
"Creative BioMart’s secretory expression platform in Bacillus delivered our amylase project ahead of schedule. The turnkey services eliminated bottlenecks in gene synthesis and strain optimization."
-Prof. Wei Zhang, PI | Enzyme Engineering Consortium
-
"Fast-tracked gene-to-protein services for COVID-19 antigen production met tight deadlines. Their expertise in large-scale fermentation and endotoxin removal (<0.05 EU/μg) ensured clinical-grade quality."
-Ms. Naomi Park, Director | Diagnostic Reagents Developer
-
"Outsourcing high-density microbial fermentation cut our client’s production costs by 35%. Real-time process analytics and scalable protein purification workflows exceeded GLP compliance requirements."
-Dr. Luca Ferrari, CSO | Biomanufacturing CDMO
FAQs
-
Q: What are the key advantages of bacterial expression systems for recombinant protein production?
A: Bacterial systems like E. coli and Bacillus subtilis offer rapid growth, cost-effectiveness, and well-established genetic tools for high-yield protein production. They are ideal for soluble cytoplasmic proteins, secretory proteins with engineered signals, and scalable industrial enzyme manufacturing.
-
Q: How does your service workflow ensure successful protein expression?
A: Our end-to-end process includes gene synthesis with host-specific codon optimization, vector design (e.g., promoter selection, affinity tags), and strain screening. For challenging targets, we apply protein refolding protocols and solubility enhancers, followed by analytical validation (SDS-PAGE, LC-MS) to guarantee quality.
-
Q: Can you handle complex proteins prone to aggregation or insolubility?
A: Yes. Our proprietary solubility optimization platform combines chaperone co-expression, redox buffer screening, and AI-driven refolding workflows to recover functional conformations from inclusion bodies. Please contact us to learn related case studies.
-
Q: What makes your bacterial systems a cost-effective alternative to mammalian platforms?
A: Bacterial fermentation achieves higher cell densities and faster turnaround times, significantly reducing protein production costs. We optimize expression vectors and growth conditions to maximize yields for therapeutic proteins, diagnostic antigens, and biocatalysts.
-
Q: Do you support large-scale production for commercial applications?
A: Absolutely. Our large-scale fermentation services (up to 20,000L bioreactors) and GMP-compliant downstream processing ensure seamless scale-up from research to commercialization. Applications include vaccine antigens, industrial enzymes, and biosimilars requiring batch-to-batch consistency.
Resources
- Protein Expression and Purification Services
- Protein Engineering Services
- Baculovirus-Insect Cell Expression
- Mammalian Expression Systems
- Membrane Proteins Expression and Purification
Contact us or send an email at for project quotations and more detailed information.
Quick Links
-

Papers’ PMID to Obtain Coupon
Submit Now -

Refer Friends & New Lab Start-up Promotions