Renaturation of Recombinant Proteins from Inclusion Bodies
Background
Critical Steps of Renaturation
Refolding recombinant proteins from inclusion bodies entails extracting proteins expressed in E. coli and restoring their bioactivity. Here's how it usually goes down:
1. Inclusion Body Isolation: Start by breaking open cells mechanically and washing using detergents or mild denaturing agents to separate the inclusion bodies.
2. Solubilizing Inclusion Bodies: Use strong denaturants like urea or guanidine hydrochloride, along with reducing agents, to dissolve the inclusion bodies.
3. Protein Refolding: This involves forming the correct disulfide bonds, which can be achieved by methods like:
- Air oxidation
- Glutathione reoxidation starting from reducing agents
- Disulfide bond exchange starting from peptides with mixed disulfides.
4. Purifying Refolded Protein: The final step involves purifying molecules with catalytic activity. The success of this phase depends on many physical factors and chemical/biological agents that determine folding time, prevent protein clumping, assist in folding, and affect the solubility and stability of the native molecules.
 Fig. 1. Integral membrane proteins in liposomes. (Singh A.; et al. Microb Cell Fact. 2015)
Fig. 1. Integral membrane proteins in liposomes. (Singh A.; et al. Microb Cell Fact. 2015)
Methods for Refolding of Proteins
Refolding proteins often takes time and involves some complexity, requiring adjustments specific to each protein. The method you choose depends on the recombinant protein's type and its physical, chemical, and biological characteristics. Common techniques for protein refolding are:
- Dialysis
- Slow dilution
- Rapid dilution
- Pulse renaturation
- Chromatography
- Ion exchange
- Gel filtration
Service Details
Service Process
What We Offer?
- Optimized Expression of Recombinant Protein
We provide Protein Expression and Purification Services with a variety of expression systems, from which you can choose the expression that suits your research needs. Optimizing recombinant protein expression is crucial for efficiently producing high-quality target proteins. We tailor and fine-tune expression vectors based on the protein's characteristics, selecting the right promoters, tags, and multiple cloning sites. We also assess various host systems, like E. coli, yeast, insect, or mammalian cells, to find the best expression platform. Systematic experiments help refine conditions like culture medium, temperature, induction timing, and inducer concentration to maximize yield and functionality. We also offer solutions for challenging proteins, such as hydrophobic ones or those with multiple disulfide bonds, for specific needs.
- Separation and purification of inclusion bodies
Isolating and purifying inclusion bodies comes with its share of challenges, like keeping the integrity of the inclusion bodies intact, thoroughly removing host impurities, and setting up the right conditions for protein refolding. We have plenty of experience and optimized methods to finely control cell lysis conditions, ensuring the inclusion bodies stay intact. Our specially designed washing protocols and buffer formulations help maintain high purity of the target protein and effectively remove interfering substances. For those tricky or hard-to-handle samples, we also provide custom solutions, like adjusting washing intensity and centrifugation settings to match the protein's properties, overcoming bottlenecks and delivering high-quality, refolded inclusion body products.
- Reduction condition optimization
We offer comprehensive support for optimizing refolding conditions, helping clients tackle common protein refolding challenges like aggregate formation, low refolding efficiency, and activity loss. Using a high-throughput screening platform, we systematically test combinations of refolding buffers, salt concentrations, pH levels, redox agents, and additives (like glycerol, urea, arginine) to identify conditions that maximize refolding. We also explore and refine various refolding strategies, such as dilution, dialysis, and stepwise refolding, tailored to different target proteins. For proteins with complex structures or specific functional needs, our team can design customized refolding processes, such as segmented refolding or multi-step adjustments, and employ online monitoring for real-time evaluation.
- Evaluation of protein activity and stability
In evaluating the activity and stability of refolded proteins, we address their unique challenges with comprehensive solutions. We accurately assess the biological activity of refolded proteins using sensitive functional tests like enzyme assays, receptor binding studies, and pathways analysis. Alongside, high-resolution structural techniques like CD spectroscopy, fluorescence analysis, and dynamic light scattering help confirm the correct native conformation. Additionally, we conduct long-term stability tests on refolded proteins, checking activity retention, structural integrity, and potential aggregation across various storage conditions.
Online InquiryWhy Choose Us?
Our team of seasoned scientists boasts extensive experience in inclusion body refolding and protein engineering, crafting effective refolding strategies tailored to each protein's unique traits.
We offer a variety of refolding techniques, including dilution, dialysis, chromatography, and the use of cofactors, to efficiently recover biologically active target proteins.
Equipped with advanced tools like HPLC, MALS, and MS, we conduct precise quality assessments of refolded proteins, analyzing purity, activity, and structural integrity.
We optimize conditions for refolding challenging inclusion body proteins, aiming to maximize both recovery rates and biological activity.
We provide end-to-end solutions, from inclusion body solubilization and refolding to activity validation, all tailored to meet specific client needs and adhere strictly to project timelines.
With years of experience, we've successfully handled thousands of inclusion body refolding projects across fields like biopharma, academic research, and industrial production.
Case Study
Case 1: Simplified Production of Bioactive TRAIL in E. coli
Researchers have found that tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) triggers apoptosis in many cancer cell lines while sparing most healthy cells, attracting interest for its antitumor potential. However, producing recombinant human TRAIL (rhTRAIL) in E. coli is challenging due to the formation of insoluble inclusion bodies (IBs). The researchers developed a straightforward method to obtain pure, bioactive rhTRAIL in E. coli, requiring only basic lab equipment and achieving high purification efficiency. This method can also be adapted for other proteins forming IBs in E. coli.
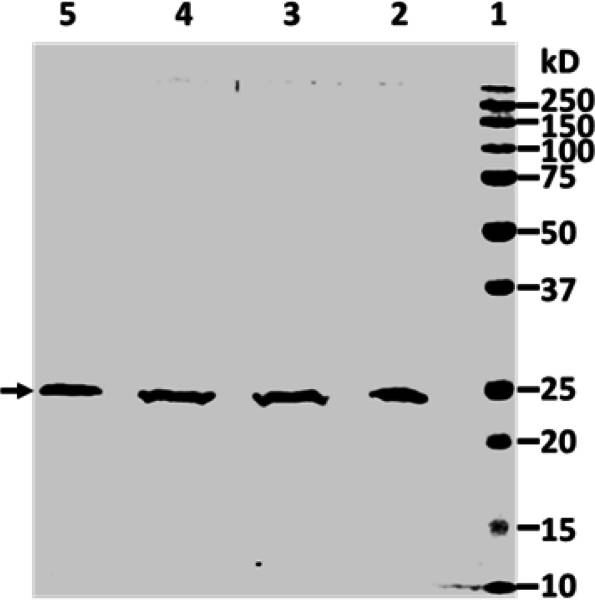 Fig. 2. Renaturing of denatured rhTRAIL. (Zhang, et al. 2020)
Fig. 2. Renaturing of denatured rhTRAIL. (Zhang, et al. 2020)Case 2: Efficient Recovery of Bioactive Proteins from Inclusion Bodies
Recovering bioactive recombinant proteins from E. coli inclusion bodies is often difficult due to poor solubilization. While urea and guanidine hydrochloride are common, they often yield low bioactive protein recovery. Mild agents like high pH buffers, detergents, and n-propanol have been successful, preserving the native-like structures in inclusion bodies. Researchers show that using 30% trifluoroethanol with 3 M urea effectively solubilizes human growth hormone inclusion bodies. This approach allows the solubilized protein to be refolded and purified, retaining activity as confirmed by cell proliferation assays. Trifluoroethanol helps maintain secondary structure while disrupting tertiary structure, proving useful for solubilizing various proteins.
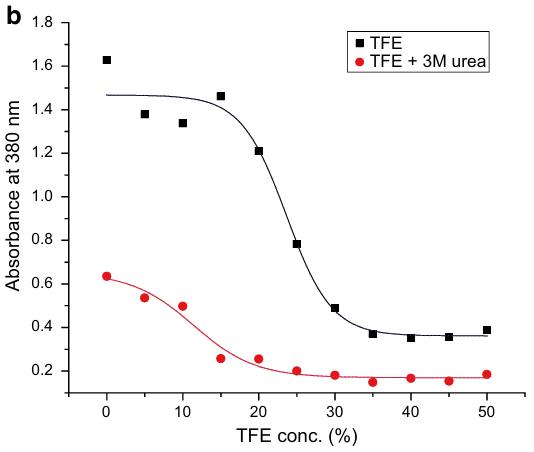 Fig. 3. Turbidimetry of hGH IBs solubilized in different concentrations of TFE in absence and presence of 3 M urea. (Upadhyay, et al. 2016)
Fig. 3. Turbidimetry of hGH IBs solubilized in different concentrations of TFE in absence and presence of 3 M urea. (Upadhyay, et al. 2016)Case 3: Mild Extraction of Spider Silk Proteins from Inclusion Bodies
Spider silk fibers, created from spidroins, are incredibly strong. Researchers commonly use E. coli to produce these proteins, which often form tough-to-dissolve inclusion bodies. Traditional methods need harsh chemicals for extraction, but this new gentle heat method efficiently dissolves these bodies. It preserves the proteins' ability to form nanoparticles using lower urea levels and controlled heating.
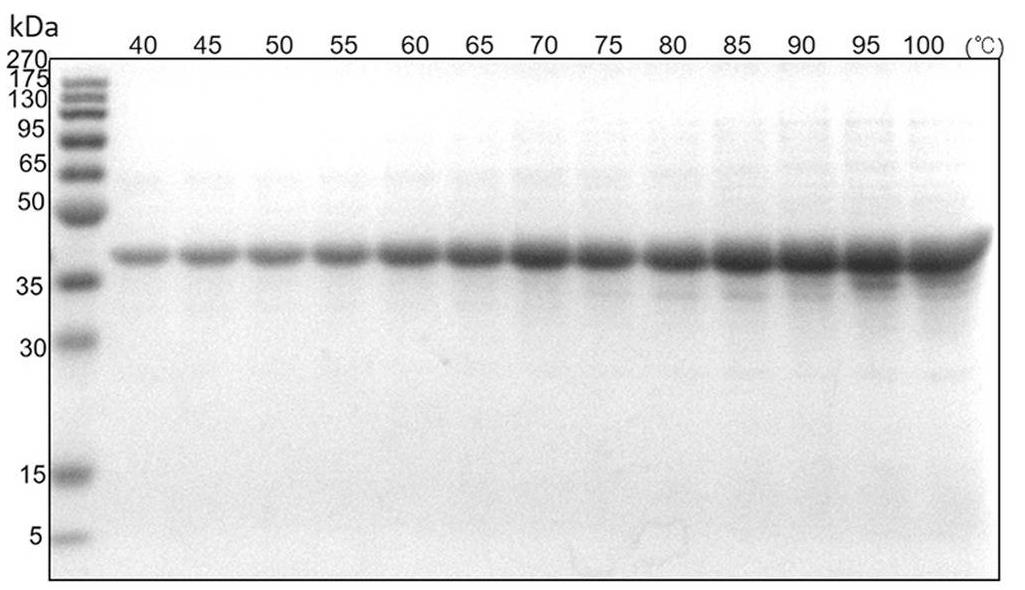 Fig. 4. SDS-PAGE analysis of the effect of heating temperature. (Cai, et al. 2020)
Fig. 4. SDS-PAGE analysis of the effect of heating temperature. (Cai, et al. 2020)FAQs
-
Q: Which proteins tend to form inclusion bodies?
A: Proteins with high expression, complex structures, or several disulfide bonds, like membrane or functional proteins, often form these bodies in foreign systems.
-
Q: Can we manage proteins with multiple disulfide bonds?
A: Absolutely, we've got well-tuned methods for optimizing disulfide bond formation to ensure they fold properly.
-
Q: Is there a guaranteed success rate for refolding?
A: Success varies with the protein, but our experienced team offers feasibility checks to boost the chances.
-
Q: How long does the service usually take?
A: It generally ranges from 2-6 weeks, depending on protein complexity and what the client needs.
References:
- Singh A.; et al. Protein recovery from inclusion bodies of Escherichia coli using mild solubilization process. Microb Cell Fact. 2015;14:41.
- Cai H.; et al. One-step heating strategy for efficient solubilization of recombinant spider silk protein from inclusion bodies. BMC Biotechnol. 2020;20(1):37.
- Zhang Z.; et al. A simplified method for the efficient purification and refolding of recombinant human TRAIL. Biotechnol Prog. 2020;36(5):e3007.
Contact us or send an email at for project quotations and more detailed information.
Quick Links
-

Papers’ PMID to Obtain Coupon
Submit Now -

Refer Friends & New Lab Start-up Promotions
