A Comprehensive Comparison of Avi-tag with Other Biotinylation Tags
Biotinylation is a powerful technique widely used in molecular biology and biochemistry for protein purification, detection, and imaging. Among various biotinylation methods and tags, the Avi-tag has gained significant attention due to its unique advantages. However, understanding its comparison with other popular biotinylation tags and methods is crucial for selecting the most appropriate tool for specific research applications.
Introduction to Biotinylation Tags
Biotinylation involves the covalent attachment of biotin to proteins or other biomolecules. The high affinity of biotin for streptavidin (Kd ~ 10⁻¹⁵ M) makes this technique extremely useful for various applications, including protein purification, detection, and imaging. Different biotinylation tags have been developed to meet diverse research needs, each with its own set of advantages and limitations.
Avi-tag: A Unique Approach to Biotinylation
 Fig1. Avi- tag structure (PBD)
Fig1. Avi- tag structure (PBD)Mechanism
The Avi-tag is a 15-amino acid sequence derived from the biotin acceptor peptide of the Bacillus subtilis biotin holoenzyme synthetase (BirA*). It enables site-specific biotinylation by the BirA* enzyme, ensuring that biotin is attached to a defined location on the protein. This specificity minimizes interference with protein function and allows for precise control over biotinylation.
Advantages
- Site-specific biotinylation: Ensures that biotin is attached to a defined location on the protein, reducing the risk of interfering with protein function.
- High efficiency: Biotinylation is highly efficient and can be performed both in vivo and in vitro, making it versatile for different experimental setups.
- Versatility: Compatible with various downstream applications, including protein purification, detection, and imaging.
- Minimal background: The specific interaction between biotin and streptavidin reduces non-specific binding, leading to cleaner results.
Limitations
- Requirement for BirA*: The system requires the presence of the BirA* enzyme, which may need to be co-expressed or added in vitro, adding an extra layer of complexity.
- Tag size: The 15-amino acid tag may be relatively large compared to other tags, potentially affecting protein folding or function in some cases.
Comparison with Other Biotinylation Tags
Biotin-LC-Lysine Tag
Mechanism
This method involves the covalent attachment of biotin to lysine residues using a long-chain linker (LC). It is commonly used for antibody labeling.
Advantages
- Flexibility: Can be attached to any lysine residue, making it suitable for a wide range of proteins.
- Commercial availability: Many biotin-LC-lysine reagents are commercially available and easy to use.
Limitations
- Non-specific labeling: Biotin can be attached to multiple lysine residues, potentially affecting protein function or causing steric hindrance.
- Variable efficiency: The efficiency of biotinylation may vary depending on the protein and lysine accessibility.
Biotin-XX Tag
Mechanism
Similar to biotin-LC-lysine, but with a shorter linker (XX). It is often used for labeling antibodies or small peptides.
Advantages
- Smaller linker: The shorter linker can reduce steric hindrance compared to longer linkers.
- Versatility: Suitable for labeling a variety of biomolecules.
Limitations
- Non-specific labeling: Like biotin-LC-lysine, it can label multiple lysine residues, potentially affecting protein function.
- Limited site-specificity: Requires careful optimization to avoid unwanted effects.
Biotin-PEG (Polyethylene Glycol) Tag
Mechanism
Biotin is attached to proteins via a PEG linker, which can improve solubility and reduce immunogenicity.
Advantages
- Enhanced solubility: PEGylation can improve the stability and solubility of proteins.
- Reduced immunogenicity: PEG can shield proteins from the immune system, making it useful for therapeutic applications.
Limitations
- Complex conjugation: The PEGylation process can be more complex and less specific compared to other methods.
- Variable efficiency: The efficiency of biotinylation may vary depending on the PEG linker length and protein structure.
Comparison with Other Protein Tags
His-tag
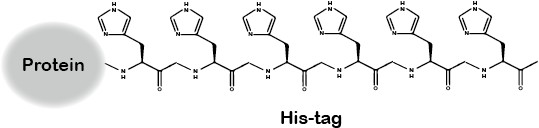
Mechanism
A polyhistidine tag (usually 6xHis) that binds to metal ions (e.g., Ni²⁺, Co²⁺) for purification.
Advantages
- Simple and efficient purification: Widely used for protein purification via immobilized metal affinity chromatography (IMAC).
- Small size: The 6xHis tag is relatively small and less likely to interfere with protein function.
Limitations
- Limited detection: Not suitable for detection or imaging applications without additional reagents.
- Non-specific binding: Can bind to other proteins with high histidine content.
FLAG-tag
Mechanism
An 8-amino acid peptide tag (DYKDDDDK) that can be detected using anti-FLAG antibodies.
Advantages
- High sensitivity: Can be detected with high sensitivity using commercial antibodies.
- Versatile applications: Suitable for protein purification, detection, and imaging.
Limitations
- Antibody dependency: Requires specific anti-FLAG antibodies, which can be expensive.
- Potential interference: The tag may interfere with protein function in some cases.
Streptavidin-Biotin System (without Avi-tag)
Mechanism
Direct biotinylation of proteins using biotinylating enzymes or chemical methods.
Advantages
- High affinity: Biotin binds to streptavidin with extremely high affinity (Kd ~ 10⁻¹⁵ M).
- Versatility: Suitable for a wide range of applications, including purification, detection, and imaging.
Limitations
- Non-specific biotinylation: Can lead to non-specific labeling, affecting protein function.
- Complex conjugation: Requires optimization for different proteins and conditions.
Summary of Key Differences
| Tag/Method | Mechanism | Advantages | Limitations |
|---|---|---|---|
| Avi-tag | Site-specific biotinylation | Site-specific, high efficiency, versatile applications | Requires BirA*, potential size interference |
| Biotin-LC-Lysine | Lysine-specific biotinylation | Flexible, easy to use | Non-specific labeling, variable efficiency |
| Biotin-XX | Lysine-specific biotinylation | Smaller linker, versatile | Non-specific labeling, potential steric hindrance |
| Biotin-PEG | PEGylated biotinylation | Enhanced solubility, reduced immunogenicity | Complex conjugation, variable efficiency |
| His-tag | Metal ion binding | Simple purification, small size | Limited detection, non-specific binding |
| FLAG-tag | Antibody-based detection | High sensitivity, versatile applications | Antibody dependency, potential interference |
| Streptavidin-Biotin (direct) | Direct biotinylation | High affinity, versatile applications | Non-specific labeling, complex conjugation |
Choosing the Right Tag
The choice of biotinylation tag or method depends on the specific requirements of your research:
- If site-specificity is crucial: Avi-tag is ideal, especially for studying protein function or structure.
- If ease of use is a priority: Biotin-LC-lysine or biotin-XX tags may be more suitable for quick labeling.
- For purification: His-tag is often preferred due to its simplicity and efficiency.
- For detection and imaging: Avi-tag or FLAG-tag can be more versatile due to their compatibility with streptavidin or specific antibodies.
- For therapeutic applications: Biotin-PEG tags may be preferred due to their enhanced solubility and reduced immunogenicity.
Selecting the appropriate biotinylation tag is essential for achieving optimal results in research applications. The Avi-tag stands out due to its site-specific biotinylation capabilities and high efficiency, making it a preferred choice for many researchers. However, other tags such as biotin-LC-lysine, biotin-XX, and His-tag also offer unique advantages depending on the specific needs of the experiment. Understanding the mechanisms, advantages, and limitations of each tag will help researchers make informed decisions and maximize the success of their experiments.
Related Products
Related Services
Related Resource
Contact us or send an email at for project quotations and more detailed information.
Quick Links
-

Papers’ PMID to Obtain Coupon
Submit Now -

Refer Friends & New Lab Start-up Promotions
