Site-Directed Mutagenesis
The GenePower TM site-directed mutagenesis service introduces single or multiple mutations (substitutions, insertions, deletions, or truncations) into existing DNA sequences quickly and economically.
Background
Introduction
Site-directed mutagenesis (SDM) is a pivotal molecular biology technique for precise DNA sequence modifications, enabling targeted nucleotide changes to revolutionize genetic engineering. Critical for protein optimization, drug discovery, and disease modeling, SDM drives advancements in therapeutic development and functional genomics. Innovations like CRISPR integration and automated platforms now enhance its efficiency and accuracy, expanding applications in vaccine design, agricultural biotechnology, and sustainable healthcare solutions. By engineering proteins with improved stability or activity, SDM addresses global challenges while accelerating biotechnological innovation.
At Creative BioMart , we specialize in delivering high-quality site-directed mutagenesis services tailored to diverse research and industrial needs. Leveraging cutting-edge technologies and a team of experienced scientists, we ensure precise, scalable, and cost-effective solutions for gene editing projects. Our commitment to rigorous quality control and rapid turnaround times positions us as a trusted partner in advancing genetic research and biotechnological applications. Ready to accelerate your research? Partner with Creative BioMart for tailored site-directed mutagenesis solutions — contact us today to discuss your project needs.
Applications
- Enzyme Engineering & Active Site Optimization
This service enables precise modifications to study and optimize enzyme active sites, supporting applications in drug development, industrial biocatalysis, or metabolic engineering. Researchers can enhance catalytic efficiency or substrate specificity for tailored bioprocesses.
- Protein Structure-Function Analysis
By introducing targeted mutations, the service clarifies how structural changes affect protein stability, ligand binding, or signaling pathways—key for antibody engineering, therapeutic protein design, or mechanistic studies in academic research.
- Gene Regulation & Nucleic Acid Interactions
The technique dissects DNA/RNA-protein binding dynamics (e.g., transcription factor-DNA interactions), aiding research in gene therapy, viral replication mechanisms, or synthetic biology circuit design.
- Synthetic Biology & Regulatory Element Tuning
Modify promoter regions or terminator sequences to fine-tune gene expression levels, optimize metabolic pathways, or engineer microbial chassis for bioproduction of pharmaceuticals and biofuels.

- Protein Tagging & Localization Studies
Construct fusion proteins (e.g., fluorescent tags) or affinity-tagged variants to streamline protein purification, track cellular localization, or analyze protein-protein interactions in live-cell imaging assays.
- Functional Genomics & Disease Modeling
Generate knockout constructs or alternative splice variants to study gene loss-of-function effects, model genetic disorders, or explore isoform-specific roles in cancer and neurodegenerative diseases.
- Therapeutic Protein Development
Perform alanine scans to map functional domains, identify drug-resistant mutations, or engineer proteins with improved therapeutic properties (e.g., antibody affinity maturation, cytokine stability).
Advantages
- Economical Alternative for Small Constructs For DNA constructs smaller than ~1 kb, site-directed mutagenesis offers a cost-efficient solution compared to synthetic gene synthesis. This approach eliminates the need for expensive oligonucleotide assembly, reducing project costs while maintaining high-quality results—ideal for academic labs and startups with budget constraints.
- Precision and Accuracy in Mutation Design The service ensures mutations are introduced exactly at specified locations without introducing unwanted secondary changes. Advanced cloning strategies and sequence-specific primers guarantee precise edits, critical for applications requiring high reproducibility, such as functional studies or regulatory-compliant therapeutic development.
- Preserved Vector Integrity Unlike PCR-based “quick change” methods, our protocols avoid amplifying the entire vector backbone. This safeguards against polymerase-induced errors in non-target regions, ensuring the original vector sequence (e.g., antibiotic resistance, replication origins) remains intact and functional.
- Large Insert Size Compatibility The technology supports inserts as large as 6-8 kb, accommodating complex constructs like multi-domain proteins, viral genomes, or synthetic pathways. This flexibility streamlines projects requiring extensive modifications without compromising cloning efficiency.
Service Details

Core Services
|
1 |
Single or Multiple Mutation Engineering |
Our service enables precise substitutions, insertions, deletions, or truncations within DNA sequences. Whether modifying a single nucleotide or introducing multiple mutations across a construct, we ensure accuracy for applications like functional genomics, protein engineering, or regulatory element analysis. |
|
2 |
Multi-Site Amino Acid Replacement |
Replace up to 7 adjacent amino acids in a single experiment to study domain interactions, modify binding epitopes, or optimize enzyme active sites. This capability supports antibody engineering, metabolic pathway tuning, and structure-function relationship studies. |
|
3 |
Flexible DNA Sequence Modifications |
Target mutations can be introduced at multiple locations simultaneously within a plasmid, reducing iterative cloning steps. This flexibility accelerates projects requiring combinatorial mutagenesis, multi-gene edits, or synthetic biology construct optimization. |
|
4 |
Truncation & Structural Editing |
Generate precise truncations to remove non-essential protein domains, disrupt regulatory sequences, or create minimal functional units. Ideal for mapping functional regions, studying splice variants, or simplifying protein purification workflows. |
|
5 |
Cost-Effective Template-Based Editing |
Modify existing DNA templates (up to 8 kb) without costly synthetic gene synthesis. This approach is optimized for constructs under 1 kb, offering rapid, economical solutions for academic labs and industrial R&D teams. |
|
6 |
Comprehensive Mutation Verification |
Our service includes comprehensive mutation verification to guarantee the accuracy and integrity of your mutated constructs. We perform full sequence validation of the insert and flanking regions using Sanger sequencing, ensuring that the desired mutations are precisely introduced without any unintended changes. |
Service Delivery & Submission Guidelines
-
- Submission Requirements
Template DNA : High-purity plasmid DNA (≥50 ng/μL) or bacterial glycerol stocks.
Mutation Details : Specify substitutions, insertions, deletions, or truncations (positions, sequences).
File Formats : Submit plasmid maps as .gb or .fasta files.
Primers (Optional) : Custom primers require HPLC purification and Tm values.
Quality Standards :- Vector size ≤8 kb.
- Avoid damaged/low-concentration DNA to prevent delays.
-
- Delivery Details
Turnaround : 5–7 business days (standard); 3 days (expedited).
Deliverables :- Mutated plasmid (10 μg, lyophilized or in solution).
- Full sequencing report (insert+flanking regions).
Storage : Free template storage for 2 years; extensions available.
Why Choose Us?
- Precision in Complex Mutations We specialize in multi-site mutations (up to 7 loci) and large DNA inserts (≤8 kb), ensuring accuracy even for technically demanding projects.
- Dual Verification Quality Control Every construct undergoes Sanger sequencing and restriction digest validation to guarantee mutation accuracy and plasmid integrity.
- Scalable High-Throughput Solutions Our optimized workflows support single constructs or bulk orders (100+ monthly), maintaining consistency for academic and industrial needs.
- Expert Technical Consultation Dedicated scientists assist with mutation design, codon optimization, and troubleshooting to align outcomes with your research objectives.
- Transparent Project Timelines Standard delivery within 5 business days (expedited: 72 hours), backed by real-time updates and a 98% on-time completion rate.
- Cost-Effective Flexibility Economical pricing for small constructs (<1 kb) and free template storage (2 years) reduce long-term project costs.
Case Study
Case 1: Engineering Tyrosine Ammonia Lyase for Neutral pH Activity
Research Background : Tyrosine Ammonia Lyase (TAL) is crucial for producing p-coumaric acid, but existing enzymes are most active at alkaline pH (9.0-10.5). Industrial processes need TAL variants that work efficiently at neutral pH.
Methods : Researchers used site-directed mutagenesis to modify the TAL enzyme from Rhodobacter sphaeroides . They changed 21 residues to reduce negative charges on the surface and increase positive charges near the active site. Single and double mutants were created and tested for activity and pH optimal shifts.
Results : Single mutants P68H, P9D, and P484E shifted the pH optimal to 8.0 and increased activity by up to 4.8-fold. The double mutant P9D/P484E shifted the pH optimal to 7.0 and increased activity sixfold at neutral pH.
Conclusion : The engineered double mutant P9D/P484E of TAL from R. sphaeroides shows high activity at neutral pH, making it suitable for industrial applications.
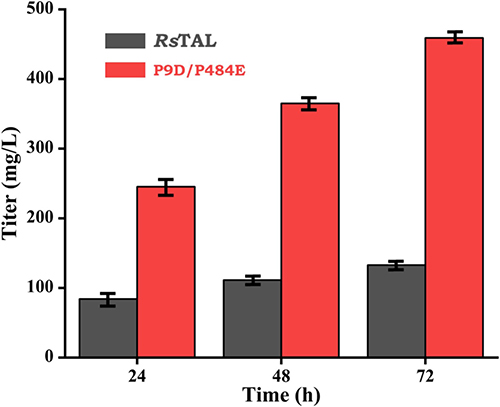
Fig1. De novo synthesis of p-coumaric acid yield was compared between wild-type RsTAL and double mutant P9D/P484E. (Iqbal, et al ., 2025)
Case 2: Enhancing Endolysin Activity Against Gram-Negative Bacteria
Research Background : Endolysins are potential alternatives to antibiotics due to their rapid bactericidal action and low resistance risk. However, their effectiveness against Gram-negative bacteria is limited by the outer membrane. This study explored how modifying the surface charge of endolysins could enhance their antibacterial activity against Gram-negative bacteria.
Methods : Researchers used sequence alignment analysis and affinity chromatography to purify endolysins. Site-directed mutagenesis was employed to modify the surface charge of the C-terminal amphipathic helices of endolysins. The antibacterial activity was assessed using turbidity reduction and viable cell counting assays.
Results : Two endolysins, LysTS3 and LysTS6, were identified with similar sequences but differing antibacterial activities. LysTS6 had higher activity against Gram-negative bacteria. By replacing specific amino acids (Ala156 and Glu160) in LysTS3 with lysine and alanine, respectively, its activity was enhanced. Similar modifications in other endolysins (LysSPN1S and LysJEP4) also increased their antibacterial activity.
Conclusion : Adjusting the surface charge of the C-terminal amphipathic helix to be more positive enhances endolysin activity against Gram-negative bacteria. This suggests that a positive surface charge is crucial for penetrating the outer membrane to reach the peptidoglycan layer.
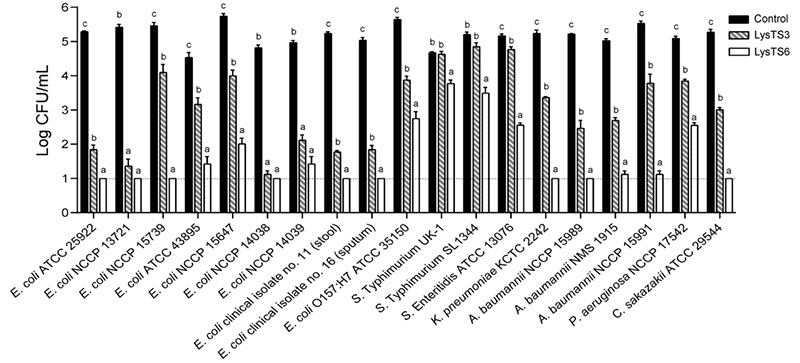
Fig2. Antibacterial spectrum of LysTS3 and LysTS6 against various Gram-negative bacteria without membrane permeabilization. (Kim, et al ., 2025)
Case 3: Linking Vitamin B6 Biosynthesis to Photosynthesis via Pyridoxine Dehydrogenase
Research Background : Vitamin B6 (VitB6) deficiency negatively impacts photosynthesis, but the underlying mechanism is not fully understood. Pyridoxine dehydrogenase (SePdx) from the cyanobacterium Synechococcus elongatus PCC 7942 is a key enzyme in VitB6 biosynthesis, and its role in photosynthesis was investigated.
Methods : The study examined the enzymatic activity of SePdx in oxidizing pyridoxine (PN) and its localization in the thylakoid membrane. Deletion of the sepdx gene and site-directed mutagenesis were used to identify key residues involved in interactions with phycobiliprotein CpcA and PSI subunit PsaE. Structural analysis and protein-protein interaction studies were conducted to elucidate these interactions.
Results : Deletion of sepdx led to distorted thylakoid membranes, reduced membrane spacing, and decreased phycobiliprotein content. SePdx was found to interact with CpcA and PsaE, and key residues mediating these interactions were identified through site-directed mutagenesis. These findings suggest that SePdx influences phycobilisome (PBS) assembly.
Conclusion : SePdx, a key enzyme in VitB6 biosynthesis, plays a role in photosynthesis by influencing PBS assembly. This study establishes a link between VitB6 biosynthesis and photosynthesis, highlighting the importance of SePdx in cyanobacterial stress responses and photosynthetic efficiency.
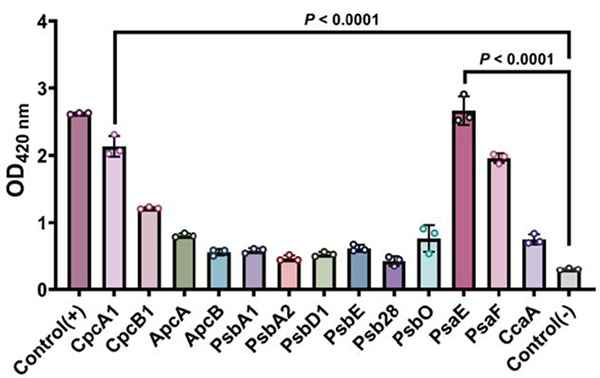
Fig3. The output from the two-hybrid system was detected as β-galactosidase activity. (Fan, et al ., 2025)
Customer Testimonials
-
"Creative BioMart’s site-directed mutagenesis service accelerated our antibody affinity maturation project. Their precision in introducing 5 simultaneous mutations streamlined our lead optimization phase. A game-changer for therapeutic development!"
-Dr. Sarah Johnson, Head of Biotherapeutics | Biopharma Innovator
-
"Modifying promoter regions in our drought-resistant gene constructs was seamless. Their ability to handle large inserts (7 kb) and deliver within 5 days exceeded expectations. Essential for agricultural biotechnology pipelines."
-Dr. Michael Chen, Agri-Biotech R&D Lead | Crop Engineering Firm
-
“Alanine scanning across 6 active sites provided crystal-clear insights into enzyme thermostability. Rigorous sequence verification ensured reliable data for patent submissions. A trusted partner for protein engineering.”
-Prof. Emma Walters, Enzyme Optimization Lead | Industrial Biocatalysis Co.
-
"Rapid CRISPR-compatible edits enabled us to prototype 20+ diagnostic variants in 3 weeks. Their multi-site mutation flexibility and cost-effective pricing supported our high-throughput COVID-19 assay development."
-Dr. Raj Patel, Molecular Diagnostics Director | Diagnostic Solutions Provider
-
"Knockout construct delivery for our metabolic pathway project was flawless. Their expertise in vector integrity preservation and template storage slashed our iterative cloning costs by 40%. Vital for synthetic biology ventures."
-Ms. Lena Müller, Synthetic Biology Lead | Biofuel Startup
-
"Customized truncations in our viral glycoprotein constructs enhanced antigenic stability. Full sequence reports and ISO-compliant workflows accelerated regulatory approvals. Unmatched precision for vaccine R&D."
-Dr. James Wu, Vaccine Development Head | Global Pharma Corp
FAQs
-
Q: What types of mutations can be introduced using your site-directed mutagenesis service?
A: Our service supports substitutions, insertions, deletions, and truncations in DNA sequences. Additionally, we enable simultaneous replacement of up to 7 adjacent amino acids in a single experiment. This versatility caters to applications like functional genomics, enzyme engineering, and structure-function studies. Whether modifying promoter regions or optimizing protein domains, our protocols ensure precise, targeted edits. -
Q: How large of an insert can your service accommodate?
A: We handle inserts as large as 6–8 kb, accommodating complex constructs such as multi-domain proteins, viral genomes, or synthetic metabolic pathways. This capability streamlines projects requiring extensive modifications, such as CRISPR-based gene editing or large-scale synthetic biology workflows, without compromising cloning efficiency. -
Q: What are the advantages of your service compared to synthetic gene construction?
A: For constructs under ~1 kb, our service provides a cost-effective alternative to synthetic gene synthesis by eliminating costly oligonucleotide assembly. It ensures precision—mutations are introduced only at specified sites—avoiding unwanted secondary changes common in synthetic methods. This approach is ideal for iterative testing, such as alanine scanning or promoter optimization. -
Q: How do you ensure vector backbone integrity during mutagenesis?
A: Unlike PCR-based “quick change” methods, our workflow avoids amplifying the vector backbone, preventing polymerase-induced errors in non-target regions. Post-mutation, constructs undergo dual verification (Sanger sequencing + restriction digest) to confirm both the desired edit and preserved vector functionality (e.g., antibiotic resistance, replication origins). -
Q: What is the turnaround time, and do you offer expedited options?
A: Standard projects are completed in 5–7 business days, while expedited services deliver results in 3 days. Our 98% on-time completion rate and real-time progress tracking ensure transparency. Delays from complex edits are communicated upfront for client approval.
Resources
References:
- Iqbal MW.; et al. Engineering the pH optimum of tyrosine ammonia lyase from Rhodobacter sphaeroides via computationally guided rational strategy. Int J Biol Macromol. Published online March 21, 2025.
- Kim J.; et al . Surface charge of the C-terminal helix is crucial for antibacterial activity of endolysin against Gram-negative bacteria. J Biomed Sci. 2025;32(1):38.
- Fan S.; et al . Pyridoxine dehydrogenase SePdx regulates photosynthesis via an association with the phycobilisome in a cyanobacterium. Plant J . 2025;121(6):e70055.
Contact us or send an email at for project quotations and more detailed information.
Quick Links
-

Papers’ PMID to Obtain Coupon
Submit Now -

Refer Friends & New Lab Start-up Promotions
