Virus-Like Particles (VLPs) Technology Platform
Background
Introduction to Virus-Like Particles (VLPs)
Virus-Like Particles, or VLPs, are quite intriguing as they imitate the structure of real viruses but lack any genetic material, so they can’t cause infections. These particles naturally come together through the assembly of viral capsid proteins, creating a structure that mirrors the three-dimensional shape and antigen aspects of the actual viruses. The standout features of VLPs include their impressive safety and ability to provoke strong immune responses, while still being modifiable in various ways. This makes them perfect for crafting new vaccines and other biotechnological uses since they can stimulate a vigorous immune reaction without the threat of viral disease.
VLPs are making waves in multiple crucial fields, showcasing significant application potential. In vaccine development , they serve as a central tool either to prevent viral infections or to create therapeutic vaccines. Their customizable surface features allow them to be used in targeted drug delivery systems , efficiently transporting active ingredients to specific cells. Furthermore, VLPs are extensively employed in imaging and diagnostic technologies , providing efficient solutions for disease monitoring and precision medicine. As research tools , they play an influential role in basic virological studies, aiding in the understanding of virus assembly and immune response mechanisms, thus contributing to scientific advancements in these fields.
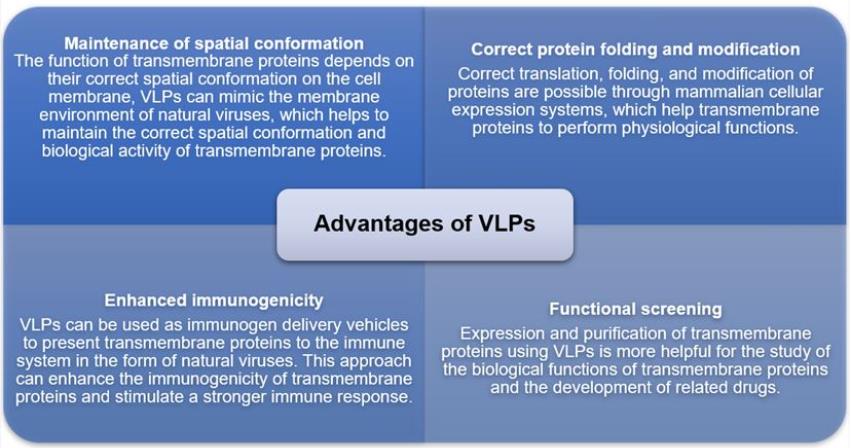
Comprehensive VLPs Technology Platform Overview
With the growing demand for safer, more effective, and customizable solutions in virus vaccines, drug delivery, and gene therapy, traditional methods are hitting some roadblocks. Traditional vaccines, for example, might carry safety risks, while drug delivery systems often struggle with precise targeting and biocompatibility. Additionally, producing VLPs can be challenging due to low expression efficiency and purification difficulties. Many existing platforms lack the flexibility and customization required for different applications, especially when it comes to constructing and functionalizing viral capsid proteins. These limitations can impede progress in developing innovative biopharmaceutical solutions.
To tackle these issues, our VLP technology platform steps up with cutting-edge research tools and multiple host systems, offering a comprehensive solution from conceptual design to large-scale production. We excel at customization, tailoring VLPs to meet specific client needs, which assures their broad application in vaccine development, targeted drug delivery, and other biopharmaceutical fields. By refining expression and purification processes, our platform overcomes bottlenecks associated with large-scale production, delivering high-purity, stable VLPs that perform reliably across various applications. Our approach guarantees both flexibility and effectiveness, making us a trusted partner in advancing biomedicine.
Service Details
Service Process
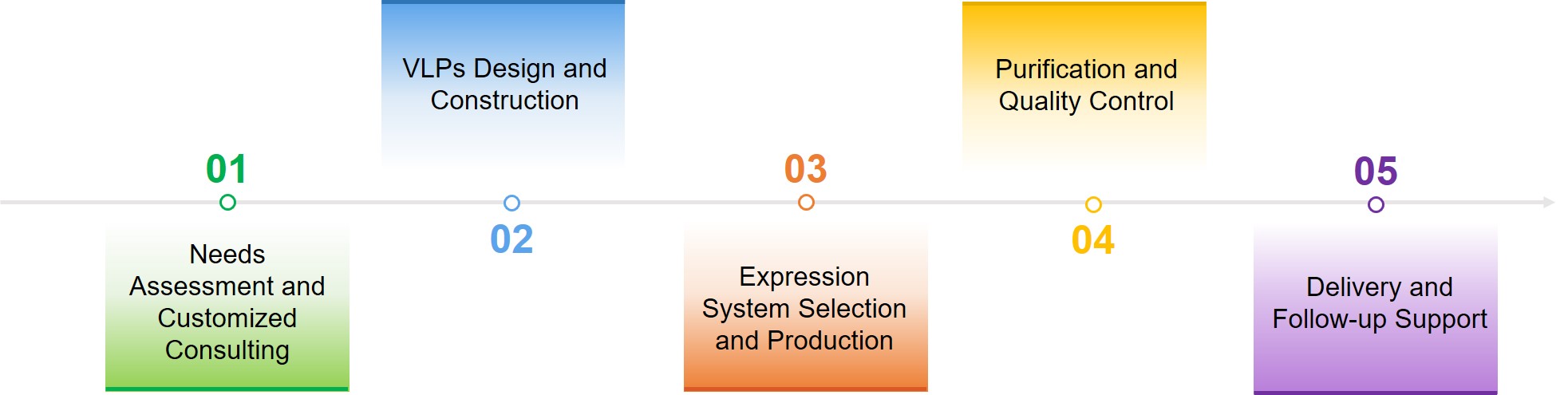
What We Offer?
|
Feasibility Study and Consultation |
VLP Design and Construction |
Host System Selection and Protein Expression |
Purification and Characterization |
Modification and Functionalization |
Scale-Up Production |
Custom Analytical Services |
|---|---|---|---|---|---|---|
|
|
|
|
|
|
|

Why Choose Us?
- Extensive Expertise : We have an extensive team of experts who are familiar with the construction and application of various virus-like particles.
- Comprehensive Solutions : Provide end-to-end services from VLP design, expression to production to meet diverse needs.
- Advanced Technology Platform : Equipped with advanced research and development equipment and optimized processes to support multi-host systems and large-scale production.
- High Customization Flexibility : Flexible customization to ensure that the specific objectives of each project are met.
- Quality and Compliance : Adhere to high quality standards, GLP/GMP production specifications, to ensure product safety and reliability.
- Seamless Project Management : Efficient project management processes ensure on-time delivery and customer satisfaction.
Case Study
Case 1: Exploring VLPs for Insulin Receptor Studies
Recent research highlights the potential of virus-like particles (VLPs) as an innovative tool for studying receptor-ligand interactions within natural plasma membrane settings. These VLPs, composed of a viral core protein enveloped by sections of the cell membrane, present receptors and membrane proteins in their natural form. In this study, researchers leveraged CHO T10 cells, which overexpress the insulin receptor (IR), to produce IR-bearing VLPs. They assessed VLP size and structure through dynamic light scattering and scanning electron microscopy. High-affinity IR presence on VLPs was confirmed using binding assays, showing parallels to IR on originating cells. Interestingly, modifying membrane cholesterol levels with agents like methyl-β-cyclodextrin notably reduced insulin binding to both cells and VLPs by over 30%. This suggests shifts in receptor positioning could explain previously observed dips in insulin signaling.
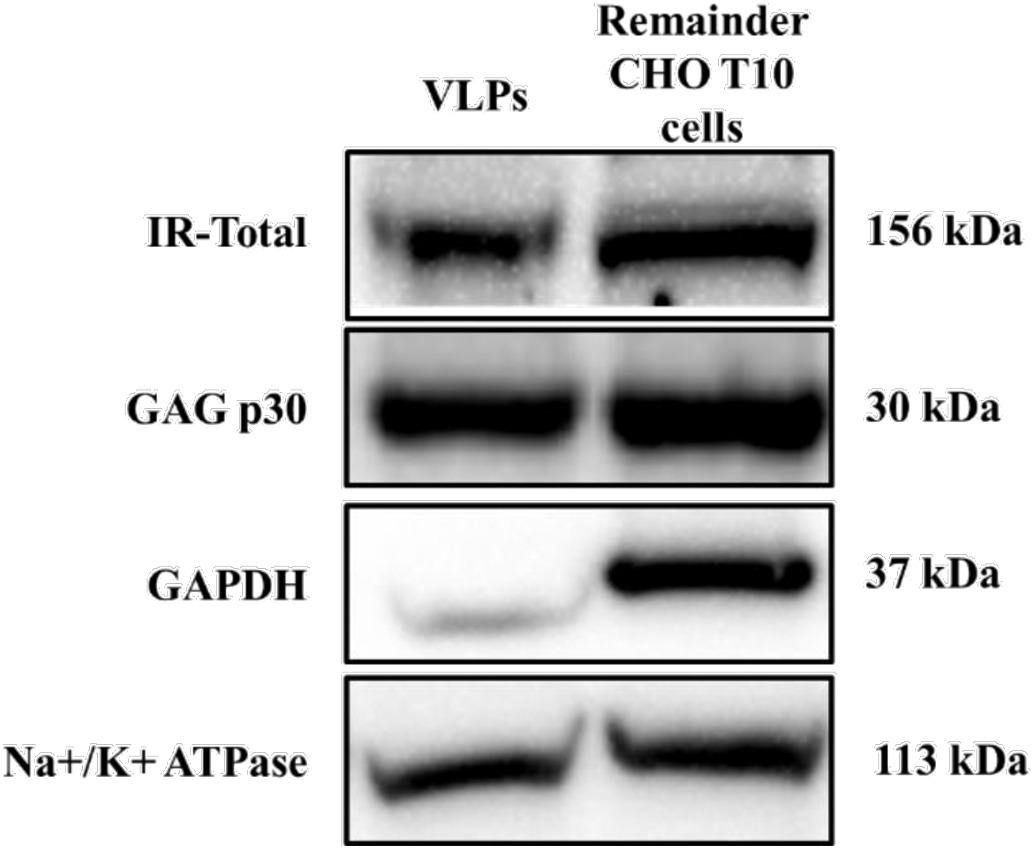
Fig. 1. Insulin receptor expression in VLPs and parental CHO T10 cells after VLP generation. (Sabapathy, et al. 2019)
Case 2: High-Speed Production of Membrane Proteins on VLPs
Conformationally complex membrane proteins (MPs) are often targeted in drug development, yet their production can be tough without efficient tools. A promising method involves co-expressing MPs with matrix proteins from enveloped viruses to maintain their native lipid environment on virus-like particles (VLPs). Researchers have utilized a site-specific recombinase-mediated cassette exchange (RMCE) strategy to create an insect cell line that expresses HIV-1 Gag for swift MP production on Gag-VLPs. Initially, the Sf9 cell line expressed a Gag-GFP cassette tagged with flipase recognition target sites (FRTs) within its fusion linker, which was later swapped for a Cherry cassette through flipase recombination. By fusing Gag with fluorescent proteins, they efficiently screened for high Gag expression while preserving the VLP framework. After recombination with Flp, the best cell clone produced Gag-VLPs adorned with a human β2-adrenergic receptor (β2AR). The presence of fluorescently labeled β2AR co-localized with Gag-VLPs was confirmed through immunoblotting and confocal microscopy, with ELISA showing their differential binding affinities to anti-β2AR antibodies.
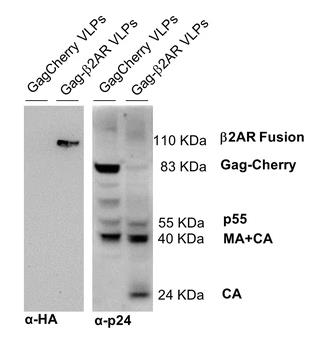
Fig. 2. Characterization of Gag-VLPs in ultracentrifuged supernatant samples from Sf9-GagCherry-11 and Sf9-Gag-β2AR cell cultures. (Vidigal, et al. 2018)
Case 3: Exploring Viral Membrane Protein Interactions with VLPs
Viral membrane proteins are key players in the virus life cycle, making them prime candidates for drug targeting. Virus-like particles (VLPs) offer a unique platform to analyze these proteins within their native environment. In this study, researchers developed VLPs containing full-length hemagglutinin (HA) or neuraminidase (NA) from influenza and explored their interactions with small molecule inhibitors. They discovered that standard purification methods left VLPs contaminated with serum proteins, hindering studies. By incorporating gel filtration chromatography, they significantly reduced these impurities. The findings showed that WaterLOGSY NMR outperformed STD NMR in analyzing ligand interactions with VLP-embedded targets. Moreover, they investigated ligand orientations using HA VLPs compared with recombinant forms. Finally, through NA-VLP analysis, they examined the binding dynamics of substrate analogs, emphasizing on the resistant H274Y-NA mutant.
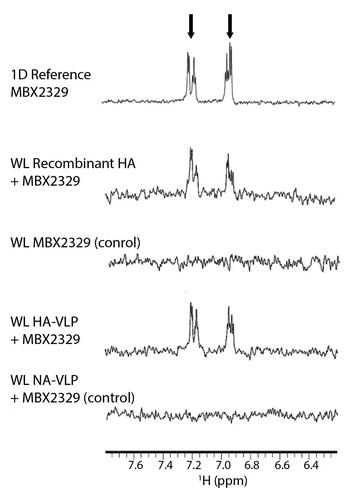
Fig. 3. WL NMR spectra of the MBX2329 interaction with recombinant HA and HA embedded in VLP. (Antanasijevic, et al. 2016)
FAQs
-
Q: How do VLPs compare to traditional cell-based membrane protein production methods in terms of efficiency?
A: Absolutely. Our platform offers highly customizable solutions tailored to your specific research and application needs. -
Q: How do you ensure the purity and stability of the produced membrane proteins?
A: We use improved methods for expressing and purifying proteins to ensure they are very pure and intact. These enhanced techniques help keep the proteins stable and active, making them useful for many different applications later on.
-
Q: Is it possible to ask for a Western Blot (WB) analysis if I don’t have my own antibodies?
A: Certainly, during the construction phase, we can incorporate a tag and later use specific tag antibodies to conduct the WB analysis.
-
Q: Am I able to request an ELISA activity test? What need I to supply?
A: With our extensive background in creating ELISA tests, once protein expression is accomplished, we can offer an additional service for ELISA testing. You should provide antibodies targeting the relevant protein and specify details such as the volume, concentration, type (like mouse or rabbit antibodies), and any other pertinent information essential for the development of the test.
References
- Sabapathy T.; et al. Use of virus-like particles as a native membrane model to study the interaction of insulin with the insulin receptor. Biochim Biophys Acta Biomembr. 2019;1861(6):1204-1212.
- Vidigal J.; et al. RMCE-based insect cell platform to produce membrane proteins captured on HIV-1 Gag virus-like particles. Appl Microbiol Biotechnol. 2018;102(2):655-666.
- Antanasijevic A.; et al. Application of virus-like particles (VLP) to NMR characterization of viral membrane protein interactions. J Biomol NMR. 2016;64(3):255-265.
Contact us or send an email at for project quotations and more detailed information.
Quick Links
-

Papers’ PMID to Obtain Coupon
Submit Now -

Refer Friends & New Lab Start-up Promotions
