TRPV1
-
Official Full Name
transient receptor potential cation channel, subfamily V, member 1 -
Overview
The transient receptor potential cation channel subfamily V member 1 (TrpV1), also known as the capsaicin receptor and the vanilloid receptor 1, is a protein that, in humans, is encoded by the TRPV1 gene. It was the first isolated member of the transient receptor potential vanilloid receptor proteins which in turn are a sub family of the transient receptor potential protein group. This protein is a member of the TRPV group of transient receptor potential family of ion channels. -
Synonyms
Trpv1;transient receptor potential cation channel, subfamily V, member 1;Vr1;Vr1l1;VR.5"sv;transient receptor potential cation channel subfamily V member 1;OTRPC1;capsaicin receptor;vanilloid receptor 1;osm-9-like TRP channel 1;vanilloid type 1 receptor;vanilloid receptor subtype 1;vanilloid receptor type 1 like protein 1
Recombinant Proteins
- Human
- Mouse
- Chicken
- Rat
- Zebrafish
- E.coli
- Mammalian Cells
- HEK293
- His
- Non
- Flag
- Strep
- TRxA
- Avi
- Fc
- GST
Background
What is TRPV1 Protein?
TRPV1, or transient receptor potential vanilloid subtype 1, is a ligand-gated ion channel that doesn’t favor any specific cation, and it’s part of the TRP channel family. You’ll mostly find it in sensory neurons of the peripheral nervous system, but it’s also present in the central nervous system and various non-neuronal tissues. It kicks into action with several triggers — think heat over 43°C, acidic conditions, or spicy compounds like capsaicin. TRPV1 is a key player in how we feel pain, regulate body temperature, and respond to inflammation. Because of its role in pain signaling, targeting TRPV1 could lead to new treatments for chronic pain, potentially moving us away from heavy reliance on opioids. Plus, it’s involved in controlling body temperature, circadian rhythms, energy intake, and even fat metabolism.What is the Function of TRPV1 Protein?
TRPV1, short for Transient Receptor Potential Vanilloid 1, is a non-selective cation channel found mostly in sensory neurons but also present in various non-neuronal cells. It acts like a multi-signal integrator, reacting to several triggers like high temperatures above 43°C, low pH levels, and capsaicin from chili peppers. When activated, it lets calcium ions into the cell, which depolarizes neurons and kicks off action potentials. This process plays a big role in how we feel pain, regulate temperature, and respond to inflammation. Furthermore, TRPV1 is involved in neuro-immune interactions, influencing immune cell functions. Given these roles, it’s seen as a promising target for treating chronic pain, cardiovascular diseases, and some types of cancer.TRPV1 Related Signaling Pathway
TRPV1 is like a versatile gatekeeper in our bodies, heavily involved in how we feel pain, regulate our temperature, and handle inflammation. When it gets triggered by stuff like high temperatures, low pH levels, or spicy capsaicin, it lets calcium ions flow into cells. This kickstarts nerve cell activity and releases neuropeptides like CGRP and Substance P, which are linked to various bodily functions and ailments. Beyond that, TRPV1 plays a part in the crosstalk between nerves and the immune system, influencing how immune cells work. The calcium signals it manages are crucial for cell actions like growth, death, and cytokine secretion. So, in the grand scheme of things, TRPV1 is key in pain signaling, inflammation, and the progression of several diseases, making it a promising target for treating chronic pain and related conditions.TRPV1 Related Diseases
TRPV1 proteins have a big role in how our bodies handle pain and inflammation, as well as other processes. They’re the main players in pathological pain, like inflammatory, neuropathic, and cancer-related pain. In heart issues, TRPV1 is involved in things like high blood pressure and heart attacks. They also connect to chronic skin conditions like psoriasis and rosacea, lung diseases like asthma, and even cognitive problems linked to diseases like Alzheimer’s. In cancer studies, boosting TRPV1 seems to help stop tumor growth and spread, making these proteins crucial in many health issues and a promising target for new treatments.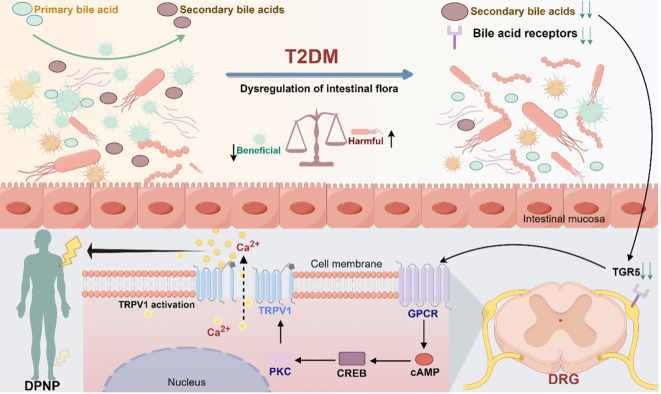
Fig1. Modulation of TGR5 receptors and TRPV1 ion channels in gut microbiota mediating diabetic peripheral neuropathic pain. (Peng Chen, 2024)
Bioapplications of TRPV1
Recombinant TRPV1 protein plays a significant role in research, industrial production, and medical studies. In the research arena, it’s leveraged to dive into its function in how we perceive pain, regulate temperature, and respond to inflammation, along with understanding various physiological and pathological processes. Moreover, scientists use it to create heterologous expression systems for studying its functional characteristics outside of the body. Industrially, recombinant TRPV1 is pivotal in crafting specific antibodies and diagnostic tools, and it serves as a crucial target in drug screening and development efforts. In the medical field, TRPV1 emerges as a promising target for tackling chronic pain, cardiovascular diseases, and even certain cancers. The use of recombinant TRPV1 protein aids in deciphering the molecular workings of these ailments and in formulating innovative treatment approaches. Hence, it’s clear that recombinant TRPV1 is vital to advancing scientific research and medical breakthroughs.Case Study
Case Study 1: Liao M. et al. Nature. 2013
TRP channels act as sensors for various signals, but understanding their response to different stimuli has been tough due to a lack of detailed structures. By using electron cryo-microscopy, we’ve mapped the structure of the mammalian TRPV1 channel at a 3.4 Å resolution, avoiding the need for crystallization and achieving side-chain clarity in membrane proteins. Similar to voltage-gated channels, TRPV1 has a four-fold symmetry around a central ion path made by transmembrane segments S5-S6. It features a wide opening outside and a short filter for selectivity. The TRP domain interacts with the S4-S5 linker, showing its role in allosteric modulation. Subunit structure is supported by interactions among cytoplasmic domains, including ankyrin repeats. These findings offer a framework for understanding TRP channel functions.-
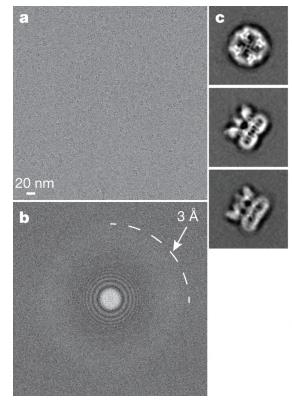 Fig1. 3D reconstruction of TRPV1 determined by single-particle cryo-EM.
Fig1. 3D reconstruction of TRPV1 determined by single-particle cryo-EM. -
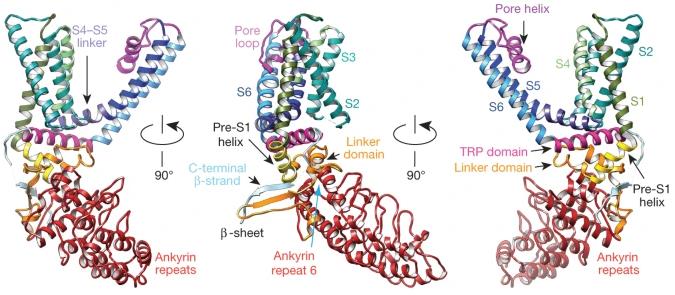 Fig2. Ribbon diagrams showing three different views of a TRPV1 monomer denoting specific domains.
Fig2. Ribbon diagrams showing three different views of a TRPV1 monomer denoting specific domains.
Case Study 2: Ohbuchi K. et al. PLoS One. 2016
So TRPV1, which is like a non-selective gate for ions, also serves as a sensor. Thanks to electron cryo-microscopy, we’ve got its precise structure, shedding light on how capsaicin and RTX bind to it. But we’re still in the dark about how other similar compounds interact. In this study, by tweaking human TRPV1 and using four chemicals—capsaicin, RTX, [6]-shogaol, and [6]-gingerol—we pinpointed amino acids key for binding and affecting proton sensitivity. Computer simulations highlighted three: L518, F591, and L670. Interestingly, [6]-gingerol seems to connect in a unique way. These findings could be a game-changer for creating TRPV1 modulators.-
 Fig3. Kinetic responses of wild-type and mutant TRPV1 in the Ca2+ flux assay.
Fig3. Kinetic responses of wild-type and mutant TRPV1 in the Ca2+ flux assay. -
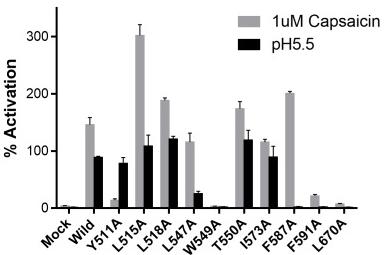 Fig4. Activation of wild-type and mutant TRPV1 channels by capsaicin and proton.
Fig4. Activation of wild-type and mutant TRPV1 channels by capsaicin and proton.
Quality Guarantee
High Purity
-
.jpg) Fig1. SDS-PAGE (TRPV1-345H)
Fig1. SDS-PAGE (TRPV1-345H)
-
.jpg) Fig2. SDS-PAGE (TRPV1-0944H)
Fig2. SDS-PAGE (TRPV1-0944H)
Involved Pathway
TRPV1 involved in several pathways and played different roles in them. We selected most pathways TRPV1 participated on our site, such as Neuroactive ligand-receptor interaction,Inflammatory mediator regulation of TRP channels, which may be useful for your reference. Also, other proteins which involved in the same pathway with TRPV1 were listed below. Creative BioMart supplied nearly all the proteins listed, you can search them on our site.
| Pathway Name | Pathway Related Protein |
|---|---|
| Inflammatory mediator regulation of TRP channels | CYP2J6,KLK1B4,PLCB4,MAPK10,PLCB2,Adcy4,PRKCH,CAMK2B,CYP2C55,IL1B |
| Neuroactive ligand-receptor interaction | CHRNA4,PRLRA,GPR156,TRHRB,TAAR14J,CHRM5A,CHRM5B,PTGER2B,CHRNB3B,P2RY11 |
-
 Fig1. TRPV1 channels in synaptic membranes are activated by lipids to influence synaptic transmission by modulating receptor activity. (Ming Fu, 2024)
Fig1. TRPV1 channels in synaptic membranes are activated by lipids to influence synaptic transmission by modulating receptor activity. (Ming Fu, 2024) -
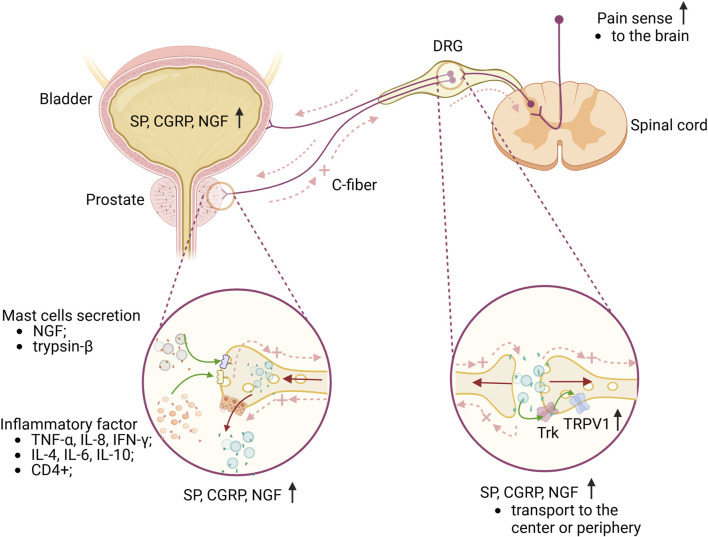 Fig2. Briefly explain the mechanism of CP/CPPS pain and lower urinary tract. (Zhipeng Jiang, 2024)
Fig2. Briefly explain the mechanism of CP/CPPS pain and lower urinary tract. (Zhipeng Jiang, 2024)
Protein Function
TRPV1 has several biochemical functions, for example, ATP binding,calcium channel activity,calcium-release channel activity. Some of the functions are cooperated with other proteins, some of the functions could acted by TRPV1 itself. We selected most functions TRPV1 had, and list some proteins which have the same functions with TRPV1. You can find most of the proteins on our site.
| Function | Related Protein |
|---|---|
| calmodulin binding | MYO5A,MYLK,STRN,SPA17,SNTB1,PPP3CC,SCN5A,PHKA1,PHKB,AKAP5 |
| calcium channel activity | TRPM2,CATSPER2,CCDC109A,TRPN1,CACFD1,TRPC5,TRPV2,PDE2A,ORAI1B,TRPV5 |
| transmembrane signaling receptor activity | SPN,OR2A4,BCAM,TLR2-1,GHRHRL,GPR113,CD3D,TNFRSF10C,KLRC3,ANTXR1 |
| calcium-release channel activity | RYR3,RYR2,JPH2,RASA3,JPH3,RYR1 |
| phosphoprotein binding | SFN,SNCA,THRAP3,APTX,ARR3,LRP11,CACNB2,SERPINB1A,PAFAH1B1,DPYSL3 |
| ATP binding | UCKL1,DCKL1B,PAPSS2B,DHX33,FGFR1B,ADCK2,ULK1,OASL,PAK1,PSKH2 |
Interacting Protein
TRPV1 has direct interactions with proteins and molecules. Those interactions were detected by several methods such as yeast two hybrid, co-IP, pull-down and so on. We selected proteins and molecules interacted with TRPV1 here. Most of them are supplied by our site. Hope this information will be useful for your research of TRPV1.
Resources
Related Services
Related Products
References
- Solorzano, C; Villafuerte, D; et al. Primary Afferent and Spinal Cord Expression of Gastrin-Releasing Peptide: Message, Protein, and Antibody Concerns. JOURNAL OF NEUROSCIENCE 35:648-657(2015).
- Ruan, Z; Wang, HM; et al. A novel caffeoyl triterpene attenuates cerebral ischemic injury with potent anti-inflammatory and hypothermic effects. JOURNAL OF NEUROCHEMISTRY 133:93-103(2015).


