G3BP1
-
Official Full Name
GTPase activating protein (SH3 domain) binding protein 1 -
Overview
This gene encodes one of the DNA-unwinding enzymes which prefers partially unwound 3-tailed substrates and can also unwind partial RNA/DNA and RNA/RNA duplexes in an ATP-dependent fashion. This enzyme is a member of the heterogeneous nuclear RNA-binding proteins and is also an element of the Ras signal transduction pathway. It binds specifically to the Ras-GTPase-activating protein by associating with its SH3 domain. Several alternatively spliced transcript variants of this gene have been described, but the full-length nature of some of these variants has not been determined. -
Synonyms
G3BP1;GTPase activating protein (SH3 domain) binding protein 1;ras GTPase-activating protein-binding protein 1;G3BP;HDH VIII;Ras GTPase activating protein SH3 domain binding protein;G3BP-1;GAP binding protein;ATP-dependent DNA helicase VIII;GAP S
Recombinant Proteins
- Human
- Zebrafish
- Chicken
- Rhesus macaque
- Mouse
- E.coli
- Mammalian Cells
- Human
- HEK293
- Wheat Germ
- In Vitro Cell Free System
- GST
- His
- Non
- Avi
- Fc
- DDK
- Myc
- Flag
Background
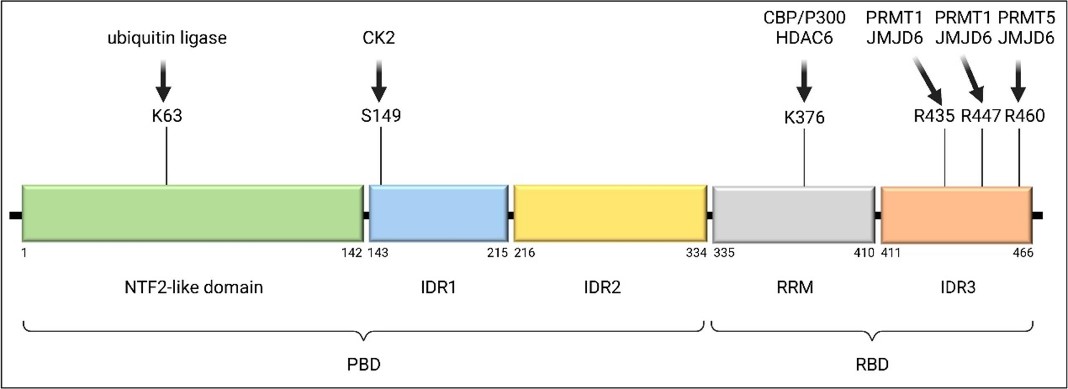
Fig1. The structure and regulation of G3BP1. (Yidong Ge, 2022)
What is G3BP1 protein?
G3BP1 (G3BP stress granule assembly factor 1) gene is a protein coding gene which situated on the long arm of chromosome 5 at locus 5q33. This gene encodes one of the DNA-unwinding enzymes which prefers partially unwound 3'-tailed substrates and can also unwind partial RNA/DNA and RNA/RNA duplexes in an ATP-dependent fashion. This enzyme is a member of the heterogeneous nuclear RNA-binding proteins and is also an element of the Ras signal transduction pathway. It binds specifically to the Ras-GTPase-activating protein by associating with its SH3 domain. The G3BP1 protein is consisted of 466 amino acids and its molecular mass is approximately 52.2 kDa.
What is the function of G3BP1 protein?
One of the most well-known functions of G3BP1 is to play a role in the assembly and dynamics of stress granules. Stress particles are cytoplasmic aggregates formed by cells in response to various stressful conditions (such as oxidative stress, nutritional deprivation, heat shock, etc.). They contain mRNA, translation initiation factors and signal transduction proteins. G3BP1 is an RNA binding protein that can interact with a variety of RNA molecules and participate in the regulation of mRNA metabolism and stability. G3BP1 interacts with RAS-GAP family proteins and is involved in regulating the activity of Ras proteins, thereby affecting cell signaling pathways. G3BP1 is involved in the regulation of cytoskeleton dynamics, affecting cell morphology and movement.
G3BP1 Related Signaling Pathway
One of the most well-known functions of G3BP1 is to play a role in the assembly and dynamics of stress granules. Stress particles are cytoplasmic aggregates formed by cells in response to various stressful conditions (such as oxidative stress, nutritional deprivation, heat shock, etc.). They contain mRNA, translation initiation factors and signal transduction proteins. G3BP1 is an RNA binding protein that can interact with a variety of RNA molecules and participate in the regulation of mRNA metabolism and stability. G3BP1 interacts with RAS-GAP family proteins and is involved in regulating the activity of Ras proteins, thereby affecting cell signaling pathways. G3BP1 is involved in the regulation of cytoskeleton dynamics, affecting cell morphology and movement. The expression and function of G3BP1 in tumor cells are related to the occurrence, development and metastasis of tumors, and are involved in many signal transduction pathways related to tumor development, including Wnt, TGF-β/Smad, PI3K/AKT/mTOR, MAPK, JAK/STAT, NF-κB, etc.
G3BP1 Related Diseases
G3BP1 acts as a cancer promoter in a variety of cancers and can promote the proliferation, invasion and metastasis of cancer cells. As an antiviral factor, G3BP1 can interact with viral proteins, regulate the assembly of stress particles, and inhibit viral replication. However, some viruses have the ability to hijack G3BP1 as a cofactor, recruiting translation primers to promote viral proliferation. G3BP1 is involved in the cellular senescence process, which it influences by controlling the senescence related secretion phenotype (SASP). In addition, it is also associated with neurological diseases, cardiovascular diseases, antiviral response.
Bioapplications of G3BP1
During drug development, the recombinant G3BP1 protein can be used as a target for drug screening, helping researchers assess the effects of potential drugs on cellular stress responses and tumor growth. Since the expression and function of G3BP1 in tumor cells are related to the occurrence, development and metastasis of tumors, the recombinant G3BP1 protein or its derivatives may be developed as drugs for the treatment of tumors. The recombinant G3BP1 protein can be used to develop diagnostic tools to help doctors assess a patient's disease state, especially in the diagnosis of tumors or diseases related to cellular stress.
Case Study
Case Study 1: Xiaomei He, 2021
RNA guanine quadruplexes (rG4) assume important roles in post-transcriptional regulations of gene expression, which are often modulated by rG4-binding proteins. Hence, understanding the biological functions of rG4s requires the identification and functional characterizations of rG4-recognition proteins. By employing a bioinformatic approach based on the analysis of overlap between peaks obtained from rG4-seq analysis and those detected in >230 eCLIP-seq datasets for RNA-binding proteins generated from the ENCODE project, the researchers identified a large number of candidate rG4-binding proteins. They showed that one of these proteins, G3BP1, is able to bind directly to rG4 structures with high affinity and selectivity, where the binding entails its C-terminal RGG domain and is further enhanced by its RRM domain. Additionally, the seCLIP-Seq data revealed that pyridostatin, a small-molecule rG4 ligand, could displace G3BP1 from mRNA in cells, with the most pronounced effects being observed for the 3'-untranslated regions (3'-UTR) of mRNAs. Moreover, luciferase reporter assay results showed that G3BP1 positively regulates mRNA stability through its binding with rG4 structures.
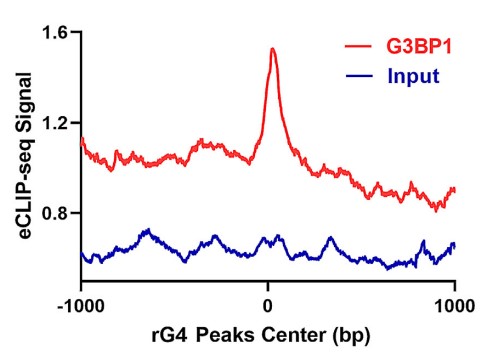
Fig1. The distribution of G3BP1 eCLIP-seq peaks relative to the center of rG4-seq peaks.
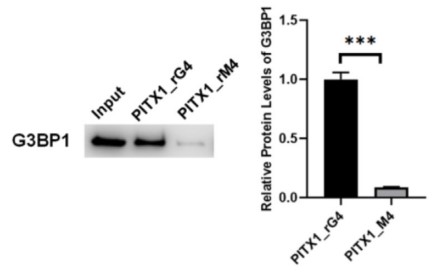
Fig2. Western blot images and quantitative results obtained from in vitro pull-down of G3BP1 protein.
Case Study 2: Amr Omer, 2020
Cellular senescence is a known driver of carcinogenesis and age-related diseases, yet senescence is required for various physiological processes. However, the mechanisms and factors that control the negative effects of senescence while retaining its benefits are still elusive. Here, the researchers show that the rasGAP SH3-binding protein 1 (G3BP1) is required for the activation of the senescent-associated secretory phenotype (SASP). During senescence, G3BP1 achieves this effect by promoting the association of the cyclic GMP-AMP synthase (cGAS) with cytosolic chromatin fragments. In turn, G3BP1, through cGAS, activates the NF-κB and STAT3 pathways, promoting SASP expression and secretion. G3BP1 depletion or pharmacological inhibition impairs the cGAS-pathway preventing the expression of SASP factors without affecting cell commitment to senescence. These SASPless senescent cells impair senescence-mediated growth of cancer cells in vitro and tumor growth in vivo.
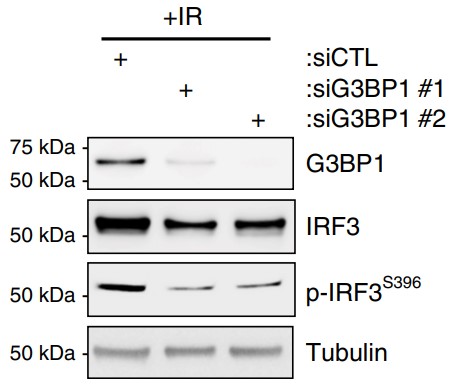
Fig3. Lysates obtained from WI-38 cells +IR cell were subjected to western blot analysis against indicated proteins.
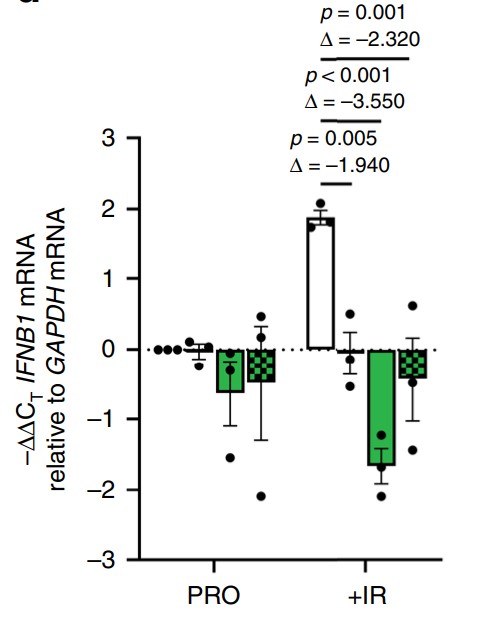
Fig4. RNA was extracted from cells treated with siRNA targeting G3BP1.
Quality Guarantee
High Purity
.jpg)
Fig1. SDS-PAGE (G3BP1-2051H)
.
.jpg)
Fig2. SDS-PAGE (G3BP1-4609H)
Involved Pathway
G3BP1 involved in several pathways and played different roles in them. We selected most pathways G3BP1 participated on our site, such as , which may be useful for your reference. Also, other proteins which involved in the same pathway with G3BP1 were listed below. Creative BioMart supplied nearly all the proteins listed, you can search them on our site.
| Pathway Name | Pathway Related Protein |
|---|
Protein Function
G3BP1 has several biochemical functions, for example, ATP binding,ATP-dependent DNA helicase activity,ATP-dependent RNA helicase activity. Some of the functions are cooperated with other proteins, some of the functions could acted by G3BP1 itself. We selected most functions G3BP1 had, and list some proteins which have the same functions with G3BP1. You can find most of the proteins on our site.
| Function | Related Protein |
|---|---|
| ATP-dependent DNA helicase activity | RTEL1,WRN,CHST13,DDX11,CHD1,XRCC6,RECQL,DHX9,CHD2,BLM |
| poly(A) RNA binding | SF3B4,RPL10A,SMC1A,ALDH6A1,EIF2S2,TMSB4X,DYNC1LI1,SF1,CCT6A,GRB2 |
| ATP-dependent RNA helicase activity | DHX32,DHX35,DHX36,DDX18,DDX54,DDX23,DDX19A,DDX25,PL10,DHX57 |
| endonuclease activity | EAR14,MRE11A,RNASE2,RNASE4,DROSHA,EME1,RNASE7,FEN1,EAR5,ANG3 |
| mRNA binding | RBM38,RBM8A,SLBP,PCF11,RPL13A,TSN,SNRNP70,SRRM4,RPS5,PURB |
| DNA binding | ZBTB2,TCEB3B,ZNF8,GCM1,PHTF2,AHRR,ZNF521,NFYA,WRN,POLR2C |
| protein binding | KRTAP12-1,THOC5,WDFY2,GM885,NR2E3,PIAS3,SPRR2A,ARHGEF6,SUMO1,FAM125B |
| ATP binding | CCT8,FARSB,ADCK2,ATP2B1,ABCA9,UBE2D1,SMYHC2,OLA1,DDX52,ABCC12 |
Interacting Protein
G3BP1 has direct interactions with proteins and molecules. Those interactions were detected by several methods such as yeast two hybrid, co-IP, pull-down and so on. We selected proteins and molecules interacted with G3BP1 here. Most of them are supplied by our site. Hope this information will be useful for your research of G3BP1.
ATXN2L;RPTOR;CSK;LCK;ATXN2;EIF4G1
Resources
Related Services
Related Products
References



