PRKAB2
-
Official Full Name
protein kinase, AMP-activated, beta 2 non-catalytic subunit -
Overview
The protein encoded by this gene is a regulatory subunit of the AMP-activated protein kinase (AMPK). AMPK is a heterotrimer consisting of an alpha catalytic subunit, and non-catalytic beta and gamma subunits. AMPK is an important energy-sensing enzyme that monitors cellular energy status. In response to cellular metabolic stresses, AMPK is activated, and thus phosphorylates and inactivates acetyl-CoA carboxylase (ACC) and beta-hydroxy beta-methylglutaryl-CoA reductase (HMGCR), key enzymes involved in regulating de novo biosynthesis of fatty acid and cholesterol. This subunit may be a positive regulator of AMPK activity. It is highly expressed in skeletal muscle and thus may have tissue-specific roles. -
Synonyms
PRKAB2;protein kinase, AMP-activated, beta 2 non-catalytic subunit;5-AMP-activated protein kinase subunit beta-2;AMPK beta 2;AMPK beta-2 chain;AMPK subunit beta-2;5-AMP-activated protein kinase, beta-2 subunit;MGC61468
Recombinant Proteins
- Human
- Mouse
- Chicken
- Rat
- Zebrafish
- E.coli
- Mammalian Cell
- HEK293
- GST
- His
- His&MBP
- Non
- His&Fc&Avi
Involved Pathway
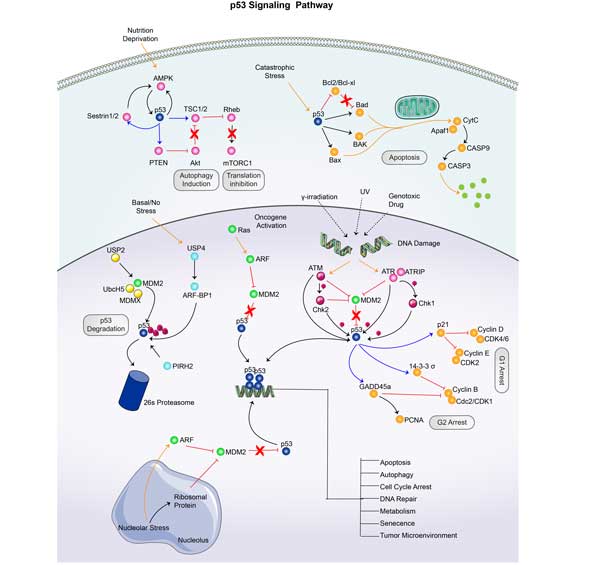
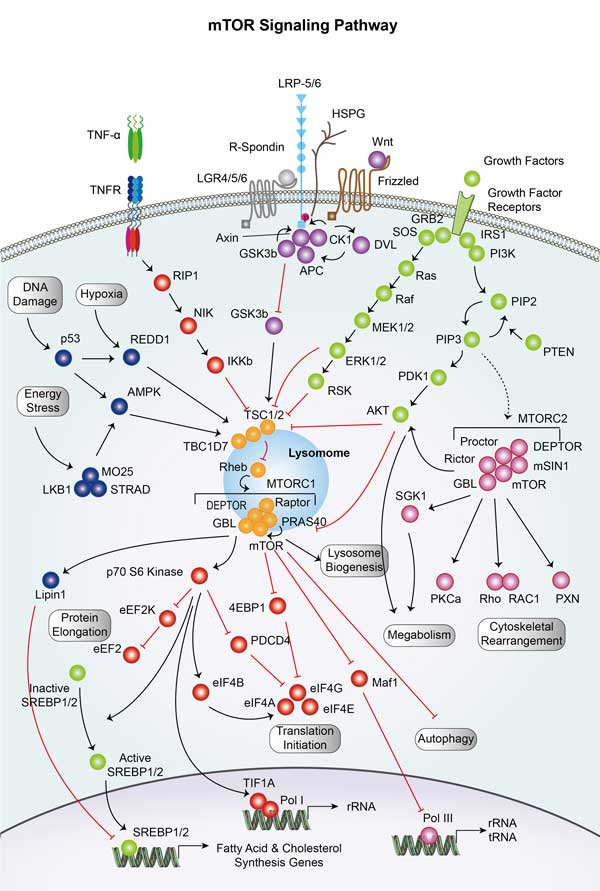
PRKAB2 involved in several pathways and played different roles in them. We selected most pathways PRKAB2 participated on our site, such as FoxO signaling pathway,AMPK signaling pathway,Circadian rhythm, which may be useful for your reference. Also, other proteins which involved in the same pathway with PRKAB2 were listed below. Creative BioMart supplied nearly all the proteins listed, you can search them on our site.
| Pathway Name | Pathway Related Protein |
|---|---|
| Adipocytokine signaling pathway | CAMKK1,CAMKK2,TNFRSF1A,POMCA,G6PCA.1,IRS4,SOCS3B,RXRB,G6PC3,RXRG |
| FoxO signaling pathway | CCND2,GABARAP,IGF1R,HOMER2,FOXO1B,MAP2K2A,TGFBR1A,PDPK1B,PIK3CB,PRKAB1 |
| Non-alcoholic fatty liver disease (NAFLD) | Fasl,KCNA3,CASP7,COX7B,LEPR,NDUFA12,NDUFA6,UQCRFS1,ERN1,PIK3R3 |
| Hypertrophic cardiomyopathy (HCM) | CACNG6,SGCB,ACTG1,ITGB3,ATP2A2,ITGA2B,MYL3,CACNB1,PRKAA2,SGCA |
| Insulin signaling pathway | PIK3CB,SOCS2,PPP1R3DA,FBP1B,G6PC3,PTPN1,RAPGEF1,MAP2K2B,AKT2,GYS1 |
| Oxytocin signaling pathway | CACNG8,MYL9,NFATC1,PIK3R3,ACTG1,MYLK3,PIK3CG,RYR1,CACNA2D4,PLCB2 |
| AMPK signaling pathway | EEF2,FOXO3,ELAVL1,INS1,PIK3CA,CREB3L4,FOXO1,CPT1A,PPP2R5C,ACACB |
| Insulin resistance | RPS6KA1,INSRA,TRIB3,PPP1R3DA,PPP1CAB,INS1,PTEN,AKT1,AKT2L,PRKAG3 |
| Circadian rhythm | RORB,PER3,PRKAA2,PRKAB1,CRY2,PER1,NPAS2,PRKAG3,NR1D1,CSNK1E |
Protein Function
PRKAB2 has several biochemical functions, for example, contributes_to AMP-activated protein kinase activity,identical protein binding,protein binding. Some of the functions are cooperated with other proteins, some of the functions could acted by PRKAB2 itself. We selected most functions PRKAB2 had, and list some proteins which have the same functions with PRKAB2. You can find most of the proteins on our site.
| Function | Related Protein |
|---|---|
| protein binding | COPS7A,CD3E,TBX5,FAM125A,PDXP,PTH2R,DOCK10,SSBP1,C11orf46,ITGA5 |
| contributes_to AMP-activated protein kinase activity | PRKAG1 |
| identical protein binding | KCNRG,GBP1,RBM10,HOOK1,LYZ2,DYNLT1A,ATF3,CASP6,TRP53,TNN |
Interacting Protein
PRKAB2 has direct interactions with proteins and molecules. Those interactions were detected by several methods such as yeast two hybrid, co-IP, pull-down and so on. We selected proteins and molecules interacted with PRKAB2 here. Most of them are supplied by our site. Hope this information will be useful for your research of PRKAB2.
PRKAA1;PRKAG1;PRKAA2
PRKAB2 Related Signal Pathway
Resources
Research Area
Kinases and Phosphatases in Endothelial CellsIntracellular Kinases in the Akt Pathway
Growth Hormone/IGF-I Axis
AMPK (AMP-activated Protein Kinases)
Related Services
Related Products
References
- Sakabe, T; Tsuchiya, H; et al. Identification of the Genes Chemosensitizing Hepatocellular Carcinoma Cells to Interferon-alpha/5-Fluorouracil and Their Clinical Significance. PLOS ONE 8:-(2013).
- Janecek, S; Svensson, B; et al. Structural and evolutionary aspects of two families of non-catalytic domains present in starch and glycogen binding proteins from microbes, plants and animals. ENZYME AND MICROBIAL TECHNOLOGY 49:429-440(2011).