SUMO-tag Protein Production
Creative BioMart offers a highly efficient, Customized SUMO-tag Protein Production Service designed to improve your recombinant protein expression outcomes. Leveraging the powerful SUMO fusion system, we enhance protein solubility and stability while enabling precise, enzymatic tag removal to yield native proteins. Whether you're working with prokaryotic or eukaryotic systems, our streamlined process delivers >90% pure protein, ready for downstream applications. We bring reliable, high-quality protein expression straight to your bench with flexible options tailored to your project needs and a fast turnaround timeline.
Background: What is SUMO-tag and Why It Matters in Protein Production
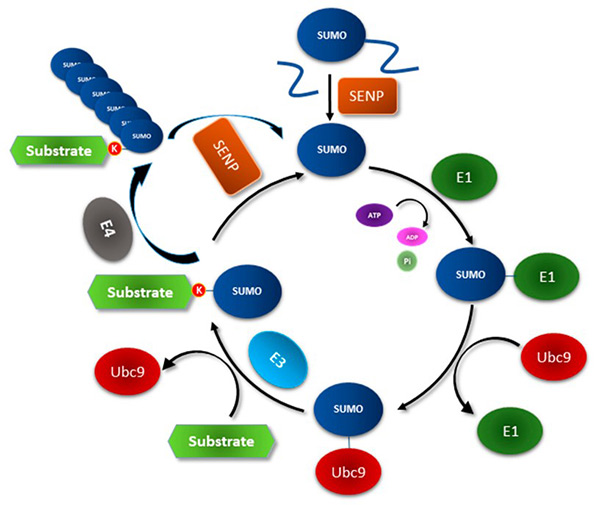
Figure 1. The Small Ubiquitin-like Modifier (SUMO) conjugation system. (Chanda et al., 2018)
Small Ubiquitin-like Modifier (SUMO) proteins are members of a highly conserved family that participate in reversible post-translational modifications. They regulate a wide range of cellular processes by forming covalent amide (isopeptide) bonds with lysine residues of substrate proteins—similar to ubiquitin, but with distinct structural and functional characteristics.
As a fusion tag, SUMO offers a unique advantage: it can markedly enhance the solubility and stability of recombinant proteins in both prokaryotic and eukaryotic expression systems. This property makes it particularly valuable for the production of proteins that are otherwise difficult to express using conventional tags.
Another significant advantage is the specificity of SUMO protease, which recognizes and cleaves the entire SUMO structure rather than a short linear sequence. This ensures precise tag removal without leaving additional residues, thereby yielding a native N-terminal protein in its authentic form.
Service Overview: SUMO-tag Protein Production Process & Details
Service Procedure

Service Details at a Glance
|
Feature |
Details |
|---|---|
|
Tag Design |
SUMO fusion at N-terminus |
|
Expression Systems |
|
|
Purification Method |
IMAC (His-tag based) |
|
Tag Removal |
SUMO protease cleavage (optional) |
|
Deliverable |
|
|
Quality Control |
SDS-PAGE, optional Western blot/mass spectrometry |
|
Typical Timeline |
8–12 weeks |
|
Customization |
Expression system, vector, scale, QC |
Advantages of Our SUMO-Tag Protein Production Services
- Enhanced Solubility & Stability: SUMO-tag improves expression yield of soluble, functional proteins—especially useful for tricky targets.
- Precise, Enzyme-specific Tag Removal: SUMO protease cleaves with surgical precision, restoring the authentic N-terminus of your protein.
- High Purity and Quality Assurance: We deliver proteins with >90% purity, validated by stringent SDS-PAGE and optional advanced assays.
- Custom-Tailored to Your Project: Every project is different—we offer flexible design options, expression hosts, and purification scales to match your research goals.
- Fast Turnaround, Transparent Communication: Typical project completion in 8–12 weeks, with clear milestones and updates along the way.
- End-to-End Support: From gene synthesis to scale-up production, we’re your one-stop shop for recombinant protein needs.
Applications of SUMO-Tag Technology in Protein Manufacturing
* NOTE: We prioritize confidentiality to safeguard our clients’ technology and intellectual property. As an alternative, we present selected published research articles as representative case studies. For details on the assay services and products used in these studies, please refer to the relevant sections of the cited literature.
Case 1: SUMO-Tagged expression of cecropin B and its bacteriolytic activity in E. coli
Park et al., 2021. doi:10.1007/s12602-021-09797-1
Antibiotic resistance has highlighted the need for alternative bacteriolytic agents like antimicrobial peptides (AMPs). Cecropin B, an AMP, was expressed in E. coli using a fusion construct with six-histidine and SUMO tags to aid purification and tag removal. The chimeric gene, codon-optimized for efficient translation, was inserted into the dual-compatible pKSEC1 vector. Among four constructs tested, the 6xHisSUMO3xGly-cecropin B with a linker showed the highest expression and efficient SUMO cleavage. Only the cleaved cecropin B demonstrated bacteriolytic activity, confirming the importance of tag removal. Optimized constructs and codon usage improve AMP expression and functionality in prokaryotic systems.
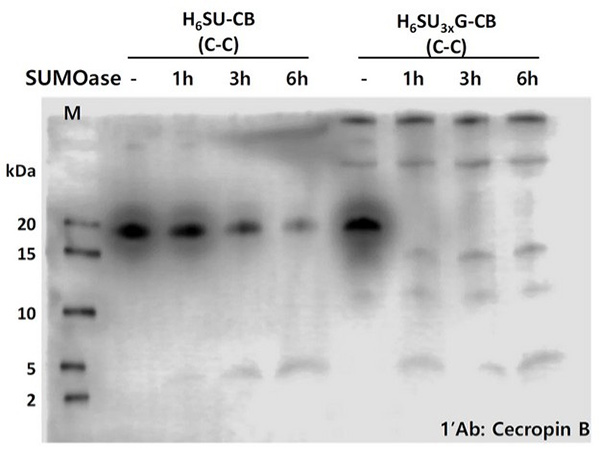
Figure 2. Western blot analysis for the comparison of expression level between 6xHisSUMO-cecropin B and 6xHisSUMO 3x Gly-cecropin B and purification of 6xHisSUMO 3x Gly-cecropin B fusion protein. Cleavage assay of the fusion proteins, 6xHisSUMO-cecropin B and 6xHisSUMO3xGly-cecropin B, by SUMOase.(Park et al., 2021)
Case 2: Enhanced expression of FMDV 3ABC protein using SUMO fusion
Zia et al., 2022. doi:10.1016/j.pep.2021.106025
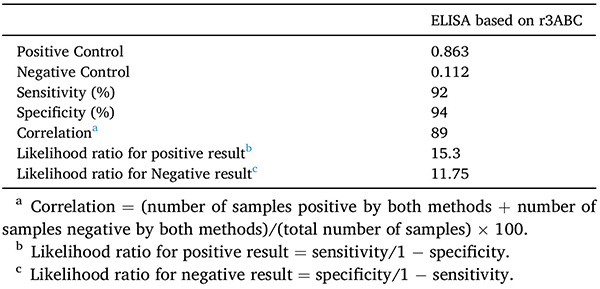
The non-structural 3ABC protein of Foot-and-mouth disease virus (FMDV) is a key diagnostic antigen for distinguishing infected from vaccinated animals (DIVA). However, its recombinant production in E. coli is challenging due to inclusion body formation and aggregation. In this study, the 3ABC gene was fused with a SUMO tag to enhance expression and solubility. Mild detergent treatment and non-denaturing Ni-NTA purification yielded 9 mg of soluble 3ABC protein per liter of culture. The recombinant protein showed strong diagnostic performance in ELISA (92% sensitivity, 94% specificity) and good thermal stability, making it a reliable antigen for FMDV serodiagnosis.
Table 1. Performance of EISA based on recombinant His-SUMO-3ABC protein. (Zia et al., 2022)
Client Reviews of Our SUMO-Tag Protein Expression Solutions
“We struggled with low solubility while expressing a zinc finger protein in E. coli , even after trying multiple tags. Creative BioMart’s SUMO-tag system finally gave us high yields of soluble, properly folded protein—without any aggregation issues. Their team also optimized the cleavage conditions, delivering a tag-free version that performed perfectly in our DNA-binding assays.”
— R&D Director | Genomics Research Institute
“For a vaccine adjuvant project, we needed high-purity recombinant flagellin expressed in a bacterial system. Creative BioMart proposed using their SUMO-tag system, which not only improved expression levels dramatically but also ensured clean tag removal. The final protein exceeded our endotoxin and purity specs for preclinical testing.”
— Principal Scientist | Immunotherapy Biotech
“We were developing a protein standard for mass spec quantification and needed native N-terminal fidelity, which most expression systems couldn’t guarantee. Creative BioMart’s SUMO-tag platform provided seamless tag removal with no extra residues, preserving the native sequence exactly. The batch reproducibility and purity were top-notch—perfect for our QC kits.”
— Project Manager | Proteomics Tools Company
“Our group needed to express a membrane-associated signaling peptide for mechanistic studies in mammalian cells, but it kept degrading. Creative BioMart suggested a SUMO fusion approach, and it worked like a charm—the fusion significantly increased peptide stability and allowed proper purification. Their customized service and technical support saved us months of troubleshooting.”
— Lab Head | Academic Molecular Biology Department
FAQs on SUMO-Tag Protein Expression and Purification
-
Q: What expression systems do you offer for SUMO-tag protein production?
A: We offer both prokaryotic ( E. coli ) and eukaryotic ( insect or mammalian ) expression systems. Our team helps select the most suitable system based on your protein’s complexity, post-translational modification needs, and downstream applications. -
Q: Can the SUMO-tag be completely removed from the final protein?
A: Yes! One of the biggest advantages of the SUMO system is that the SUMO protease cleaves with high specificity at the junction of the SUMO tag and your target protein—resulting in a native N-terminus with no extra amino acids left behind. -
Q: What are the benefits of using a SUMO-tag over other fusion tags?
A: SUMO-tag offers superior protein solubility and stability, especially for difficult-to-express or aggregation-prone proteins. It also allows for precise tag removal, giving you a clean, native-form protein ideal for functional assays, structural studies, or therapeutic applications. -
Q: How pure will the final protein product be?
A: We typically deliver protein at >90% purity, verified by SDS-PAGE. Additional quality control such as Western blot or mass spectrometry is available upon request. -
Q: Can I customize the service to fit my specific project needs?
A: Absolutely. We specialize in custom-tailored services—from vector design and expression host selection to tag removal and scale-up options. Just let us know your target protein and application, and we’ll build a solution around it. -
Q: What kind of deliverables will I receive?
A: You’ll receive purified recombinant protein, a detailed QC report, and optional aliquoting or formulation services upon request. We also retain backups for reorders or troubleshooting.
Resources
Related Services
- Mammalian Expression Systems
- Yeast Expression Systems
- Baculovirus-Insect Cell Expression
- Bacterial Expression Systems (E. coli / Bacillus)
- Protein Expression and Purification Services
- Fc Fusion Protein Production
- Albumin Fusion Protein Production Services
Related Products
References:
- Chanda A, Sarkar A, Bonni S. The sumo system and TGFβ signaling interplay in regulation of epithelial-mesenchymal transition: implications for cancer progression. Cancers. 2018;10(8):264. doi:10.3390/cancers10080264
- Park AR, Kim SW, Kim SY, Kwon KC. Expression of antimicrobial peptide (AMP), cecropin B, in a fused form to SUMO tag with or without three-glycine linker in Escherichia coli and evaluation of bacteriolytic activity of the purified AMP. Probiotics & Antimicro Prot. 2021;13(6):1780-1789. doi:10.1007/s12602-021-09797-1
- Zia MA, Shah MS, Shafqat Ali Khan R, Farooq U, Shafi J, Habib M. High level expression and purification of recombinant 3ABC non-structural protein of foot-and-mouth disease virus using SUMO fusion system. Protein Expression and Purification. 2022;191:106025. doi:10.1016/j.pep.2021.106025
Contact us or send an email at for project quotations and more detailed information.
Quick Links
-

Papers’ PMID to Obtain Coupon
Submit Now -

Refer Friends & New Lab Start-up Promotions

