SLITRK Family
Related Symbol Search List
Immunology Background
Background
The SLITRK family consists of a group of proteins known for their roles in neural development and synaptic function. Here's an overview of their origin, structure, and function:
Origin
- Discovery: The SLITRK family was first identified through studies focused on proteins involved in neural development. The name "SLITRK" derives from its association with two known protein families: SLIT proteins (which are involved in axon guidance) and TRK proteins (which are receptor tyrosine kinases associated with neurotrophic factors).
- Gene Expression: Members of the SLITRK family are predominantly expressed in the nervous system, suggesting their roles in neural tissue and potentially in other tissues as well.
Structure
Domain Composition: SLITRK proteins typically contain:
- An N-terminal signal peptide facilitating secretion.
- Multiple leucine-rich repeats (LRRs), which are involved in protein-protein interactions, suggest their role in mediating cellular communication.
- A single transmembrane domain that anchors the proteins to the cell membrane.
- A C-terminal domain that may have intracellular signaling capabilities.
Homology: SLITRK proteins show similarity to both SLIT and TRK proteins, indicating a potential dual role in signaling and cell adhesion.
Function
- Neurite Outgrowth: SLITRK proteins are crucial for promoting neurite outgrowth during development, helping to guide growing axons to their appropriate targets.
- Synaptic Function: They play a role in synapse formation and maintenance, impacting synaptic plasticity, which is essential for learning and memory.
- Role in Disorders: Dysregulation of SLITRK proteins has been associated with various neurodevelopmental disorders, including autism spectrum disorders and schizophrenia. This highlights their importance in normal neural function and development.
- Cell Adhesion and Signaling: By mediating interactions between neurons and glial cells, SLITRK proteins facilitate proper neural circuit formation and stability.
- Potential Therapeutic Targets: Given their significant roles in neural development and disorders, SLITRK proteins are being investigated as potential therapeutic targets for neurodevelopmental and neuropsychiatric conditions.
In summary, the SLITRK family of proteins is integral to neural development, influencing neurite outgrowth, synapse formation, and potential neurodevelopmental disorders, highlighting their importance in both fundamental neuroscience and clinical research.
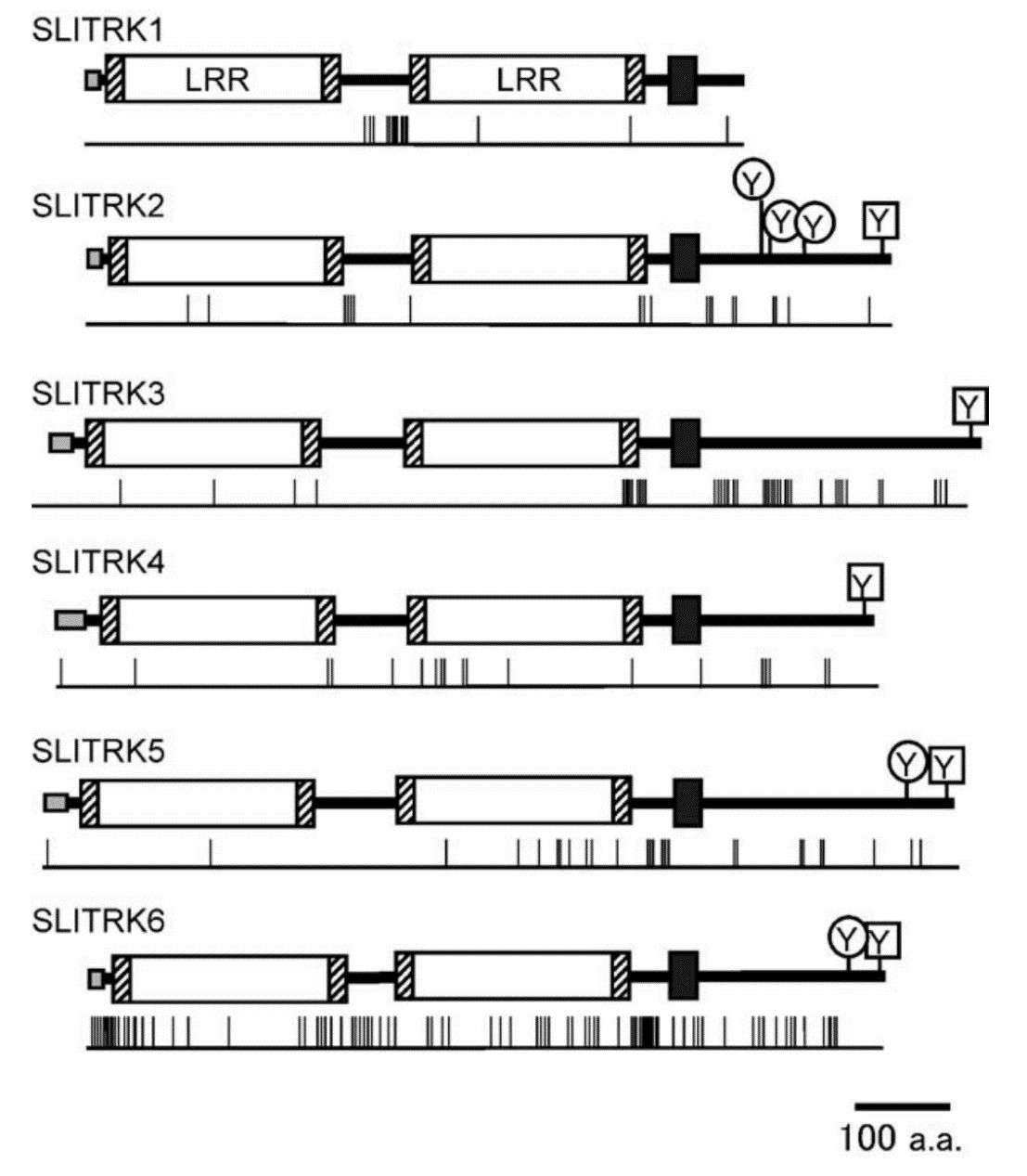 Fig.1 Structure of human SLITRK proteins. (Aruga J, et al., 2003)
Fig.1 Structure of human SLITRK proteins. (Aruga J, et al., 2003)Members of the SLITRK Family
The SLITRK family consists of six known members, each designated by a specific number. Here's an overview of the key members of the SLITRK family:
| Members | Details |
|---|---|
| SLITRK1 |
Function: Involved in neural development and synaptic functions; has been linked to neurodevelopmental disorders. Expression: Primarily expressed in the brain, particularly in areas related to cognition and emotion. |
| SLITRK2 |
Function: Associated with synapse formation and neuronal connectivity; implicated in learning and memory processes. Expression: Expressed in the central nervous system, especially in the hippocampus. |
| SLITRK3 |
Function: Plays a role in synaptic plasticity; mutations are associated with various neurodevelopmental disorders. Expression: Found in the brain and may influence neuronal network formation. |
| SLITRK4 |
Function: Thought to be involved in the development of the nervous system and the regulation of synaptic transmission. Expression: Less well characterized, but expressed in the central nervous system. |
| SLITRK5 |
Function: Plays a potential role in synaptic maturation and may influence the excitatory/inhibitory balance in neurons. Expression: Expressed in various regions of the brain. |
| SLITRK6 |
Function: Involved in neuron development and synaptic function; could be linked to certain neuropsychiatric conditions. Expression: Detected in both the central and peripheral nervous systems. |
General Features of the SLITRK Family
- Structure: Members typically contain a single transmembrane domain and multiple immunoglobulin-like and thrombospondin-like motifs.
- Signaling: SLITRK proteins may interact with receptors and other signaling molecules to modulate neuronal growth and synaptic activity.
- Clinical Relevance: Alterations in SLITRK family members have been associated with various neurological and psychiatric disorders, including autism spectrum disorders, schizophrenia, and developmental delays.
Understanding the SLITRK family is essential for unraveling the complexities of neuronal connectivity and related disorders.
Role of SLITRK Family in the Nervous System
The SLITRK family of proteins plays a crucial role in the development and function of the nervous system. Here's an overview of the importance of the SLITRK family in shaping neural circuits and neuronal communication:
Synapse Formation and Function
SLITRK proteins are key regulators of synapse formation and function in the nervous system. They participate in the development of synaptic connections between neurons, influencing the strength and plasticity of these connections.
Neuronal Development
SLITRK family members contribute to various aspects of neuronal development, including axon growth, dendritic branching, and the formation of neural circuits. They play a vital role in establishing proper neuronal connectivity during brain development.
Regulation of Neural Plasticity
SLITRK proteins are involved in modulating neural plasticity, which is essential for learning and memory processes. They help regulate the strength and efficacy of synaptic transmission, influencing how information is processed and stored in the brain.
Neurotransmitter Release
Some SLITRK family members are implicated in the regulation of neurotransmitter release at synapses. They influence the release of neurotransmitters such as glutamate and GABA, which are essential for neuronal communication.
Neurological Disorders
Mutations in SLITRK genes have been associated with various neurological disorders, including Tourette syndrome and obsessive-compulsive disorder (OCD). Dysregulation of SLITRK proteins can disrupt normal synaptic function and neural circuitry, leading to the manifestation of neurological symptoms.
Behavioral Regulation
SLITRK family members have been linked to the regulation of behaviors related to mood, anxiety, and social interactions. They play a role in shaping neural circuits that control emotional and social behaviors, influencing how individuals respond to their environment.
Signal Transduction
SLITRK proteins may participate in intracellular signaling pathways that regulate neuronal activities, such as synaptic plasticity, neuronal excitability, and cell signaling cascades crucial for neuronal function.
Understanding the intricate roles of the SLITRK family in neural development and function is essential for deciphering the mechanisms underlying brain development, synaptic plasticity, and the pathophysiology of neurological disorders. Further research on SLITRK proteins promises insights into novel therapeutic strategies for addressing neurological conditions associated with disruptions in the SLITRK signaling pathway.
Role of the SLITRK Family in Different Diseases
The SLITRK family of proteins is known to play diverse roles in various diseases, particularly neurological disorders. Here are some of the ways in which the SLITRK family is implicated in different diseases:
Neurodevelopmental Disorders
- Tourette Syndrome: a neurodevelopmental disorder characterized by repetitive, involuntary movements and vocalizations.
- Obsessive-Compulsive Disorder (OCD): a mental health disorder marked by obsessive thoughts and repetitive behaviors.
- For example, mutations in SLITRK1 have been linked to Tourette syndrome and OCD. These conditions are characterized by repetitive behaviors, tics, and intrusive thoughts. Variants in SLITRK1 are thought to disrupt synaptic function in the basal ganglia, contributing to the manifestation of symptoms.
Autism Spectrum Disorders (ASD)
A complex neurodevelopmental condition characterized by challenges with social skills, communication, and repetitive behaviors. Studies have suggested potential links between SLITRK family members and ASD. For example, SLITRK2 is thought to play a role in synapse formation and function, impacting neural connectivity and communication in the brain.
Psychiatric Disorders
SLITRK family members have been implicated in various psychiatric disorders, including anxiety disorders and mood disorders, highlighting their role in regulating emotional and behavioral responses. For example, Variants in SLITRK4 may impact neural circuits involved in emotional regulation, contributing to heightened anxiety responses. SLITRK5 has been implicated in mood disorders such as depression and bipolar disorder. Dysregulation of SLITRK5 expression may affect neurotransmitter release and synaptic function, influencing mood-related behaviors.
Neurological Conditions
Epilepsy: a neurological disorder characterized by recurrent seizures. Some SLITRK proteins are associated with epilepsy. For example, dysregulation of SLITRK6 expression may affect neuronal excitability and contribute to seizure activity.
Neuropathies: Mutations in SLITRK genes have been linked to certain neuropathies, affecting nerve function and leading to sensory or motor deficits. For example, SLITRK3 is involved in neuronal development and may play a role in maintaining nerve function.
Neurodegenerative Diseases
Emerging research suggests potential roles for SLITRK family members in neurodegenerative diseases such as Alzheimer's and Parkinson's disease. Disruptions in SLITRK signaling pathways may contribute to neuronal dysfunction and degeneration in these conditions.
Cancer
Aberrant expression of SLITRK proteins has been observed in certain cancers, suggesting potential roles in tumor growth, invasion, and metastasis. Further research is needed to elucidate the precise mechanisms by which the SLITRK family may contribute to cancer progression. For example, aberrant expression of SLITRK6 has been observed in certain cancers, including neuroblastoma. SLITRK6 may play a role in tumor growth, invasion, and metastasis, highlighting its potential as a therapeutic target in cancer treatment.
Immune Disorders
SLITRK proteins have been implicated in immune-related disorders, such as autoimmune diseases, where dysregulation of SLITRK signaling may influence immune cell function and contribute to disease pathogenesis.
Understanding the involvement of the SLITRK family in various diseases provides valuable insights into the molecular mechanisms underlying these conditions. Further research into the roles of SLITRK proteins in disease pathology may offer new avenues for therapeutic interventions and the development of targeted treatments for these disorders.
Case Study
Case 1: Chu R, Lu Y, Fan X, et al. Changes in SLITRK1 level in the amygdala mediate chronic neuropathic pain-induced anxio-depressive behaviors in mice. J Integr Neurosci. 2024;23(4):82.
Background: The co-occurrence of chronic neuropathic pain (NPP) and anxio-depressive disorders (ADD) has emerged as a significant global public-health concern. The SLIT and NTRK-like 1 (SLITRK1) protein, crucial for synaptic remodeling, exhibits high expression in the amygdala, a key brain region involved in diverse emotional behaviors. The investigation aimed to assess the involvement of amygdalar SLITRK1 protein in NPP and comorbid ADD.
Methods: A chronic NPP mouse model was induced through L5 spinal nerve ligation, with subsequent evaluation of chronic pain and ADD-like behaviors via behavioral assessments. Immunofluorescence and Western blot analyses were conducted to monitor changes in SLITRK1 protein and key proteins associated with excitatory synaptic function in the amygdala. Adeno-associated virus was employed to enhance SLITRK1 expression in excitatory synaptic neurons within the amygdala.
Results: Successful induction of chronic NPP-related ADD-like behavior was achieved in mice following L5 ligation. Decreased expression of SLITRK1 and key excitatory synaptic functional proteins like Homer1, postsynaptic density protein 95 (PSD95), and synaptophysin was observed in the amygdala in response to chronic NPP and associated ADD. Virally mediated up-regulation of SLITRK1 in the amygdala led to notable alleviation of chronic NPP and ADD symptoms, alongside restoration of Homer1, PSD95, and synaptophysin expression levels.
Conclusion: The study outcomes underscore the pivotal role of amygdalar SLITRK1 in chronic pain and related ADD, suggesting its potential as a therapeutic target for addressing the comorbidity of chronic NPP and ADD.
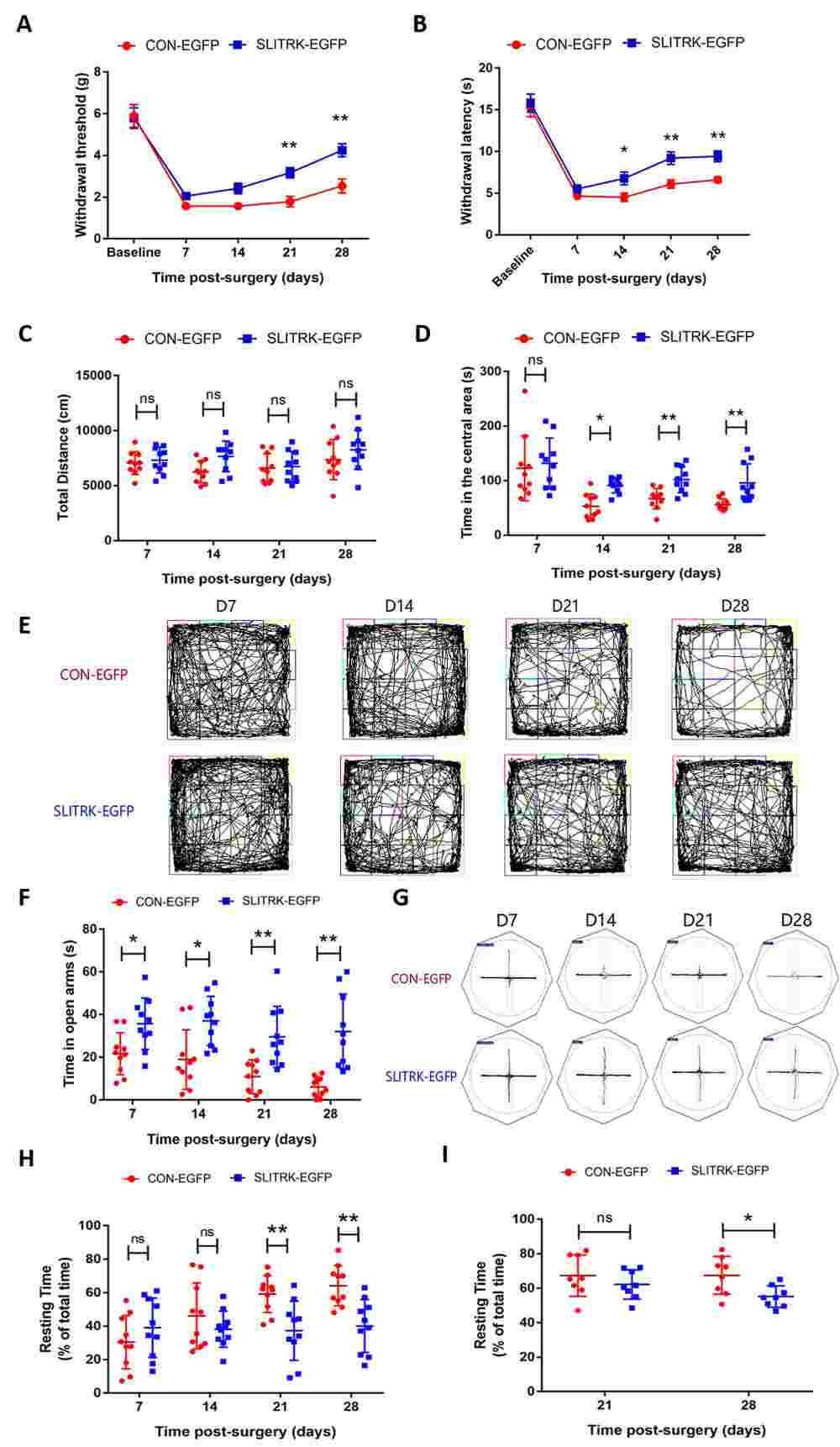 Fig.1 Effects of SLITRK1 overexpression on chronic pain and anxio-depressive disorders (ADD) comorbidities in spinal nerve ligation (SNL) mice.
Fig.1 Effects of SLITRK1 overexpression on chronic pain and anxio-depressive disorders (ADD) comorbidities in spinal nerve ligation (SNL) mice.Case 2: Matsumoto Y, Miwa H, Katayama KI, et al. Slitrk4 is required for the development of inhibitory neurons in the fear memory circuit of the lateral amygdala. Front Mol Neurosci. 2024;17:1386924.
In this study, the researchers delved into the physiological role of the Slitrk4 protein within the Slitrk family, which comprises six synaptic adhesion molecules known for their ties to neuropsychiatric disorders. By utilizing Slitrk4 knockout (KO) mice, they sought to unravel the specific functions of Slitrk4.
The researchers discovered widespread distribution of the Slitrk4 protein throughout the brain, with notable abundance in regions such as the olfactory bulb and amygdala. Through a comprehensive behavioral assessment, male Slitrk4 KO mice displayed heightened fear memory acquisition during classical fear conditioning tests and exhibited deficiencies in social behavior within reciprocal social interaction tests.
In an electrophysiological examination conducted on amygdala slices, Slitrk4 KO mice demonstrated amplified long-term potentiation in the thalamo-amygdala afferents alongside reduced feedback inhibition. Molecular marker analysis of Slitrk4 KO brains revealed a decline in the number of calretinin (CR)-positive interneurons within the anterior segment of the lateral amygdala nuclei during adulthood.
Through in vitro experiments focusing on neuronal differentiation, it was observed that Slitrk4-deficient embryonic stem cells struggled to prompt the development of GABAergic interneurons. These cells exhibited an altered response to sonic hedgehog signaling activation crucial for generating subsets of GABAergic interneurons.
The findings underscore the significance of Slitrk4 in the maturation of inhibitory neurons within the fear memory circuit. This insight not only enhances our comprehension of neuropsychiatric conditions but also sheds light on posttraumatic stress disorder, a condition where dysregulated Slitrk4 expression has been noted.
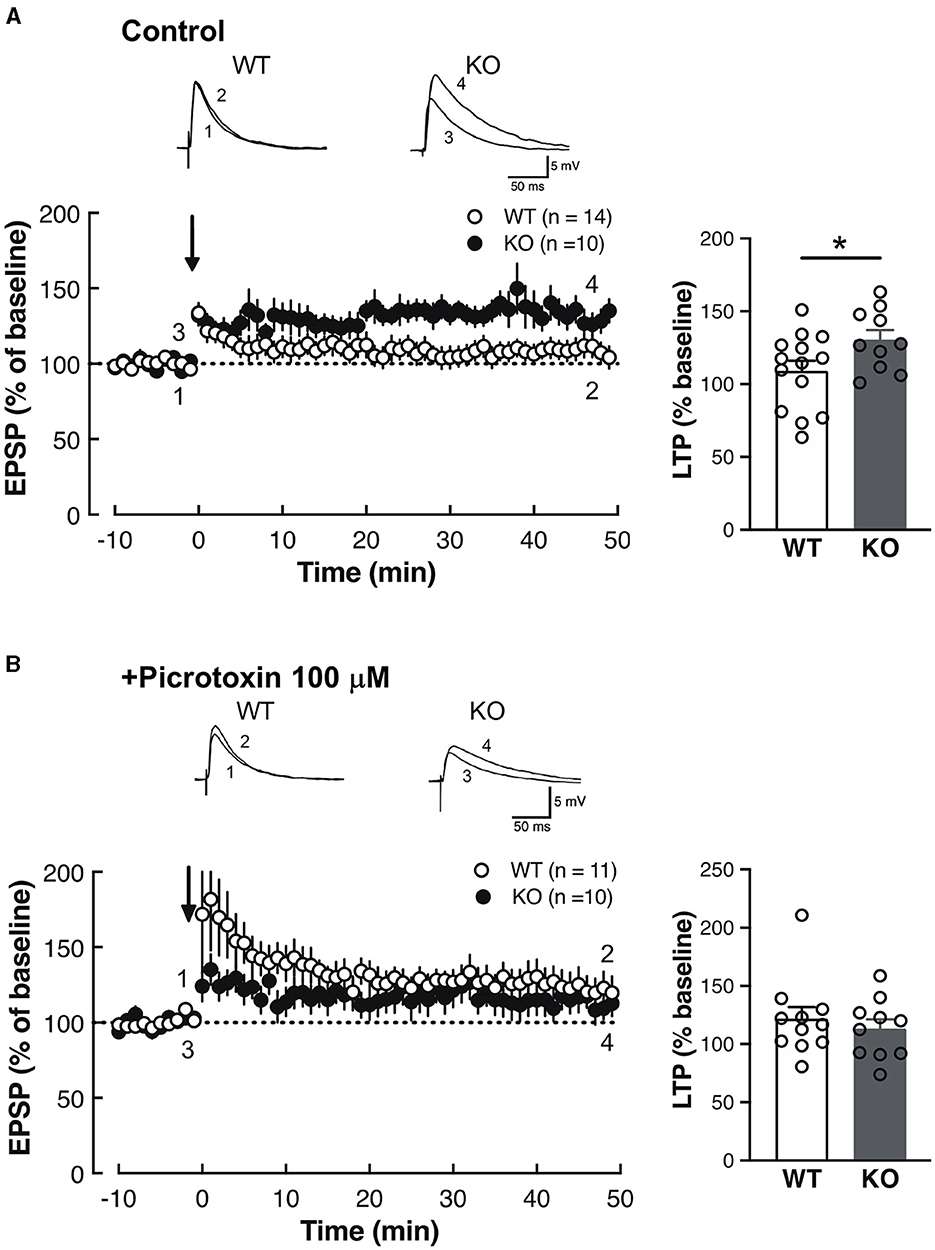 Fig.2 Enhanced synaptic plasticity in the LA of Slitrk4 KO mice.
Fig.2 Enhanced synaptic plasticity in the LA of Slitrk4 KO mice.Related References
- Zhang K, Feng Y, Wigg KG, Sandor P, Barr CL. Association study of the SLITRK5 gene and Tourette syndrome. Psychiatr Genet. 2015;25(1):31-34.
- Proenca CC, Gao KP, Shmelkov SV, Rafii S, Lee FS. Slitrks as emerging candidate genes involved in neuropsychiatric disorders. Trends Neurosci. 2011;34(3):143-153.
- Aruga J, Mikoshiba K. Identification and characterization of Slitrk, a novel neuronal transmembrane protein family controlling neurite outgrowth. Mol Cell Neurosci. 2003;24(1):117-129.
- Yim YS, Kwon Y, Nam J, et al. Slitrks control excitatory and inhibitory synapse formation with LAR receptor protein tyrosine phosphatases. Proc Natl Acad Sci U S A. 2024 Jun 18;121(25):e2410441121.
