NFkB Pathway
Creative BioMart NFkB Pathway Product List
Immunology Background
Background
The Nuclear Factor kappa-light-chain-enhancer of activated B cells (NFκB) pathway is a central signaling cascade that plays a critical role in regulating the immune response, inflammation, cell survival, and proliferation. Since its discovery in 1986, NFκB has been recognized as a key transcription factor involved in the expression of genes that control a wide range of biological processes. Dysregulation of the NFκB pathway has been implicated in several diseases, including cancer, chronic inflammation, and autoimmune disorders.
NFκB Family Members
NFκB refers to a family of transcription factors that consist of five members in mammals: RelA (p65), RelB, c-Rel, NFκB2 (p52/p100), and NFκB1 (p50/p105). These proteins share a Rel homology domain (RHD) that mediates DNA binding, dimerization, and interaction with inhibitory IκB proteins. NFκB proteins form homo- or heterodimers, with the p65/p50 heterodimer being the most studied and widely expressed complex. The NFκB dimers are typically sequestered in the cytoplasm in an inactive state by IκB (Inhibitor of κB) proteins, which include IκBα, IκBβ, IκBε, IκBγ, Bcl-3, and IκBξ.
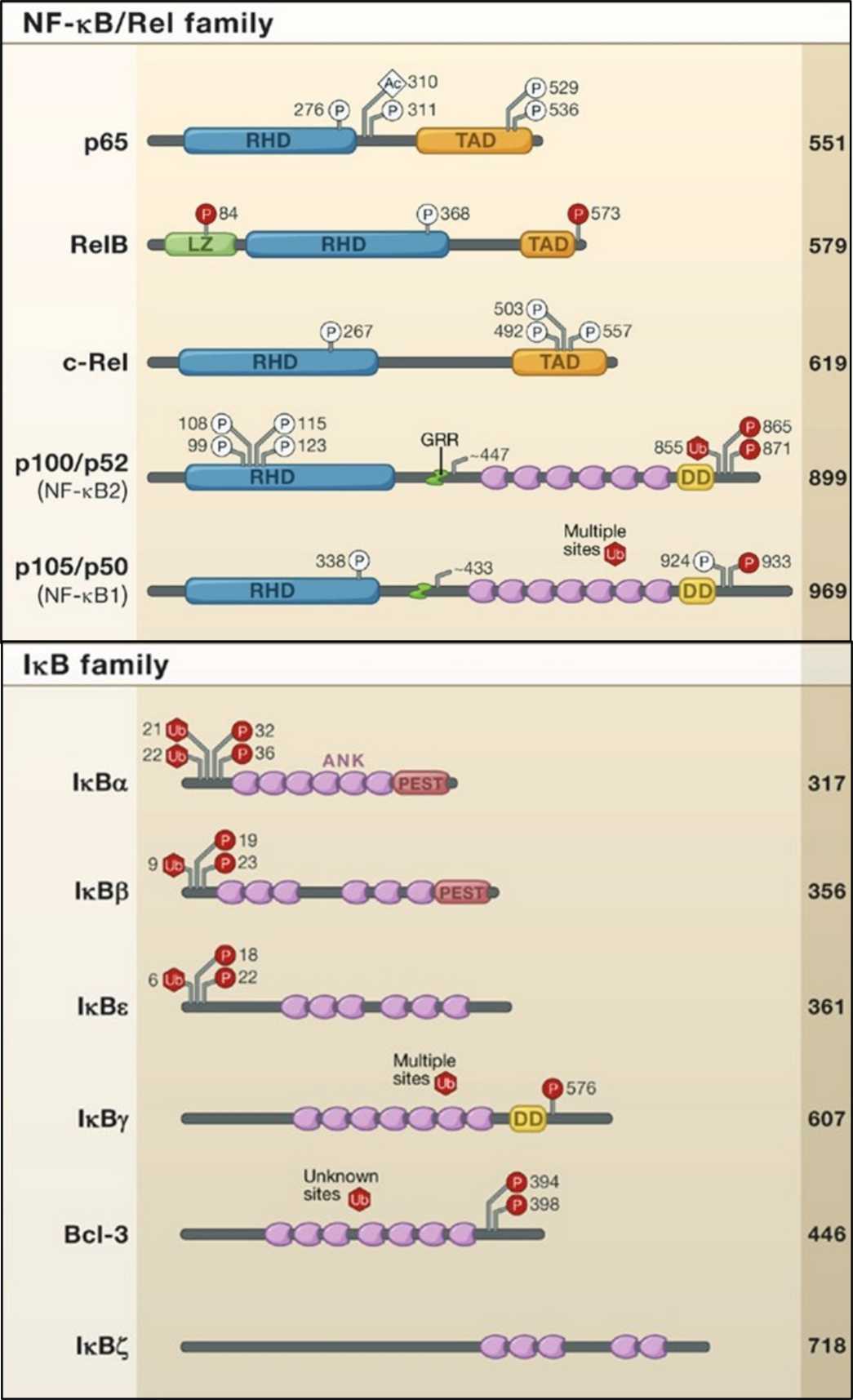 Fig. 1: Members of the NFκB and IκB proteins are shown. The number of amino acids in each human protein is indicated on the right. Posttranslational modifications that influence IKK activity or transcriptional activation are indicated with P, U, or Ac for phosphorylation, ubiquitination, or acetylation, respectively. RHD, Rel homology domain TAD, transactivation domain LZ, leucine zipper domain GRR, glycine-rich region HLH, helix-loop-helix domain Z, zinc finger domain CC1/2, coiled-coil domains NBD, NEMO-binding domain DD, death domain (Hayden and Ghosh, 2008).
Fig. 1: Members of the NFκB and IκB proteins are shown. The number of amino acids in each human protein is indicated on the right. Posttranslational modifications that influence IKK activity or transcriptional activation are indicated with P, U, or Ac for phosphorylation, ubiquitination, or acetylation, respectively. RHD, Rel homology domain TAD, transactivation domain LZ, leucine zipper domain GRR, glycine-rich region HLH, helix-loop-helix domain Z, zinc finger domain CC1/2, coiled-coil domains NBD, NEMO-binding domain DD, death domain (Hayden and Ghosh, 2008).Canonical and Non-Canonical Pathways
The NFκB signaling pathway can be broadly divided into two distinct signaling cascades: the canonical (classical) pathway and the non-canonical (alternative) pathway. Both pathways converge on the activation of NFκB transcription factors but are triggered by different stimuli and involve different molecular mechanisms.
Canonical Pathway
The canonical NFκB pathway is activated primarily by pro-inflammatory cytokines, such as tumor necrosis factor-alpha (TNF-α) and interleukin-1 (IL-1), and by pattern recognition receptors (PRRs), such as Toll-like receptors (TLRs), which recognize pathogen-associated molecular patterns (PAMPs). Upon ligand binding, these receptors initiate a signaling cascade that leads to the activation of the IκB kinase (IKK) complex, which consists of two catalytic subunits, IKKα and IKKβ, and a regulatory subunit, NEMO (NFκB essential modulator).
IKKβ phosphorylates IκB proteins, marking them for ubiquitination and subsequent degradation by the 26S proteasome. Degradation of IκB proteins releases NFκB dimers, particularly the p65/p50 heterodimer, which then translocate to the nucleus. Once in the nucleus, NFκB binds to specific κB sites in the promoter regions of target genes, leading to transcriptional activation of genes involved in inflammation, immune response, cell proliferation and survival. Key target genes of the canonical pathway include pro-inflammatory cytokines (e.g., IL-6, TNF-α), chemokines (e.g., CXCL8/IL-8), and anti-apoptotic proteins (e.g., Bcl-2, c-IAP).
Non-Canonical Pathway
The non-canonical NFκB pathway is activated by a variety of stimuli, including members of the TNF receptor superfamily such as CD40, B-cell activating factor (BAFF) receptor, and lymphotoxin-β receptor (LTβR). This pathway is dependent on the NFκB-inducing kinase (NIK) and IKKα, but independent of IKKβ and NEMO. Activation of the non-canonical pathway involves the stabilization of NIK, which phosphorylates and activates IKKα. Activated IKKα then phosphorylates the NFκB2 precursor protein p100, leading to its processing into the active p52 subunit.
The p52 subunit forms a heterodimer with RelB, and this complex translocates to the nucleus to regulate the expression of genes involved in lymphoid organ development, adaptive immune responses, and the maintenance of immune homeostasis. The non-canonical pathway is more tightly regulated and plays a role in specific immune responses rather than broad inflammatory processes.
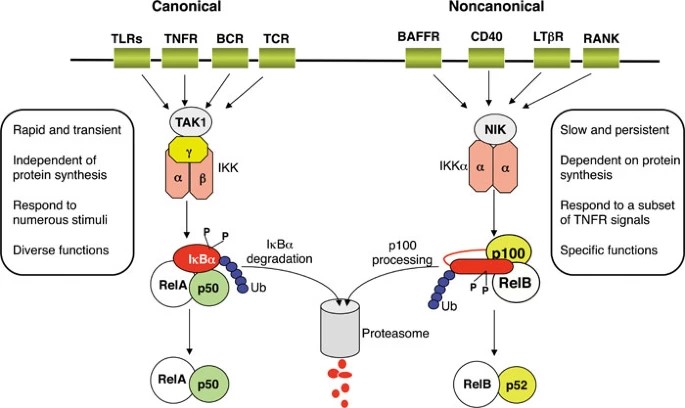 Fig. 2: Canonical and non-canonical NFκB signaling pathways (Sun, 2011).
Fig. 2: Canonical and non-canonical NFκB signaling pathways (Sun, 2011).Crosstalk Between NFκB Signaling and Other Signaling Pathways
NFκB signaling interacts, or "crosstalks," with several other signaling pathways to influence a wide range of cellular processes. Crosstalk between NFκB and other signaling pathways ensures a coordinated response to diverse stimuli, influencing processes such as inflammation, immunity, cell survival, and apoptosis. This crosstalk can be context-dependent, varying between cell types and conditions, and plays a critical role in maintaining cellular homeostasis and responding to pathological conditions such as cancer and chronic inflammation. Here's how it crosstalks with some key pathways:
MAPK Pathway: The Mitogen-Activated Protein Kinase (MAPK) pathway is involved in cell proliferation, differentiation, and stress responses. The NFκB and MAPK pathways can be co-activated by the same stimuli (e.g., cytokines or stress signals), resulting in synergistic or antagonistic effects on gene expression. For example, NFκB can induce the expression of MAPK pathway components, while MAPKs can modulate NFκB activity by phosphorylating its subunits or IκB proteins, which regulate NFκB nuclear translocation.
PI3K/Akt Pathway: The PI3K/Akt signaling pathway is critical for cell survival and metabolism. Activation of PI3K leads to activation of Akt, which promotes cell growth and inhibits apoptosis. Akt can directly phosphorylate and activate IKK (IκB kinase), which then activates NFκB by degrading IκB proteins. Conversely, NFκB can upregulate anti-apoptotic genes that are also targets of the PI3K/Akt pathway, thereby enhancing cell survival signals.
JAK/STAT Pathway: The Janus Kinase (JAK)/Signal Transducer and Activator of Transcription (STAT) pathway is involved in the response to cytokines and growth factors, regulating immune function and cell growth. NFκB can induce the expression of cytokines that activate the JAK/STAT pathway. Additionally, STAT proteins can interact with NFκB to regulate the transcription of target genes, sometimes in a cooperative manner.
Wnt/β-Catenin Pathway: The Wnt/β-catenin pathway is important in embryonic development and cancer by regulating cell fate and proliferation. The NFκB and Wnt/β-catenin pathways can regulate each other. For example, NFκB can promote the expression of Wnt pathway inhibitors such as Dkk1, while β-catenin can interact with NFκB subunits to modulate its transcriptional activity.
TGF-β/SMAD Pathway: The Transforming Growth Factor-beta (TGF-β) pathway, through SMAD proteins, regulates cell growth, differentiation, and immune responses. TGF-β signaling can inhibit NFκB activity by promoting the expression of inhibitory IκB proteins. Conversely, NFκB can induce the expression of SMAD7, an inhibitor of TGF-β signaling, thereby creating a feedback loop.
Notch Pathway: The Notch signaling pathway controls cell differentiation and plays an important role in development and cancer. NFκB can regulate the expression of Notch receptors and ligands, thereby influencing the outcome of Notch signaling. Conversely, activated Notch can interact with NFκB to modulate the transcription of inflammatory genes.
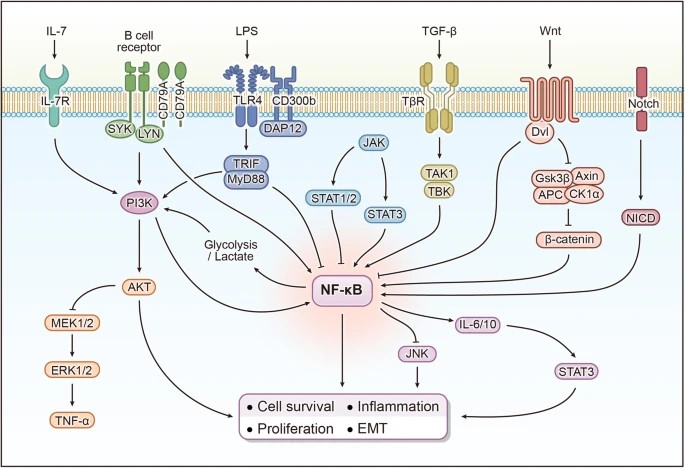 Fig. 3: The crosstalk between NFκB signaling and other signaling pathways (Guo et al., 2024).
Fig. 3: The crosstalk between NFκB signaling and other signaling pathways (Guo et al., 2024).Physiological Roles of NFκB
NFκB is a master regulator of the immune system, orchestrating the expression of genes involved in both innate and adaptive immunity. In innate immunity, NFκB controls the production of pro-inflammatory cytokines and chemokines that recruit and activate immune cells, such as macrophages and neutrophils, to sites of infection or injury. In adaptive immunity, NFκB is critical for the activation, proliferation, and differentiation of lymphocytes, including T cells and B cells.
Beyond immune responses, NFκB also plays a crucial role in cell survival and proliferation. By inducing the expression of anti-apoptotic genes and cell cycle regulators, NFκB promotes cell survival in response to stress signals, such as DNA damage or oxidative stress. This function is particularly important in the context of tissue repair and regeneration, where controlled NFκB activation ensures the survival and proliferation of cells required for tissue healing.
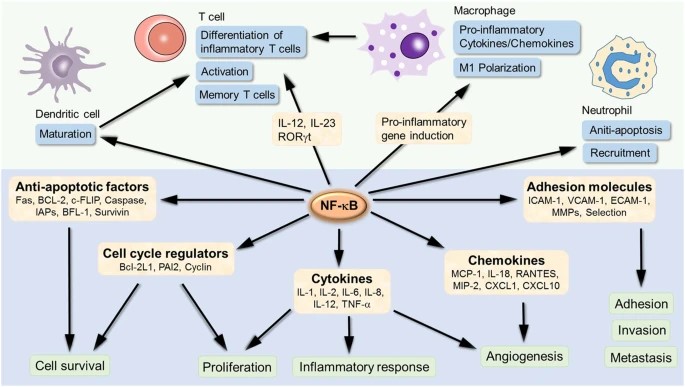 Fig. 4: NFκB target genes involved in the development and progression of inflammation. NFκB is an inducible transcription factor. Once activated, it can activate the transcription of various genes and thereby regulate inflammation. NFκB not only directly targets inflammation by increasing the production of inflammatory cytokines, chemokines and adhesion molecules, but also regulates cell proliferation, apoptosis, morphogenesis and differentiation (Liu et al., 2017).
Fig. 4: NFκB target genes involved in the development and progression of inflammation. NFκB is an inducible transcription factor. Once activated, it can activate the transcription of various genes and thereby regulate inflammation. NFκB not only directly targets inflammation by increasing the production of inflammatory cytokines, chemokines and adhesion molecules, but also regulates cell proliferation, apoptosis, morphogenesis and differentiation (Liu et al., 2017).Pathological Implications of NFκB Dysregulation
While NFκB is essential for normal immune function and tissue homeostasis, its dysregulation is associated with a wide range of diseases. Chronic activation of the NFκB pathway is a hallmark of many inflammatory and autoimmune disorders, such as rheumatoid arthritis, inflammatory bowel disease, and asthma. In these conditions, persistent NFκB activation leads to excessive production of pro-inflammatory cytokines, contributing to tissue damage and disease progression.
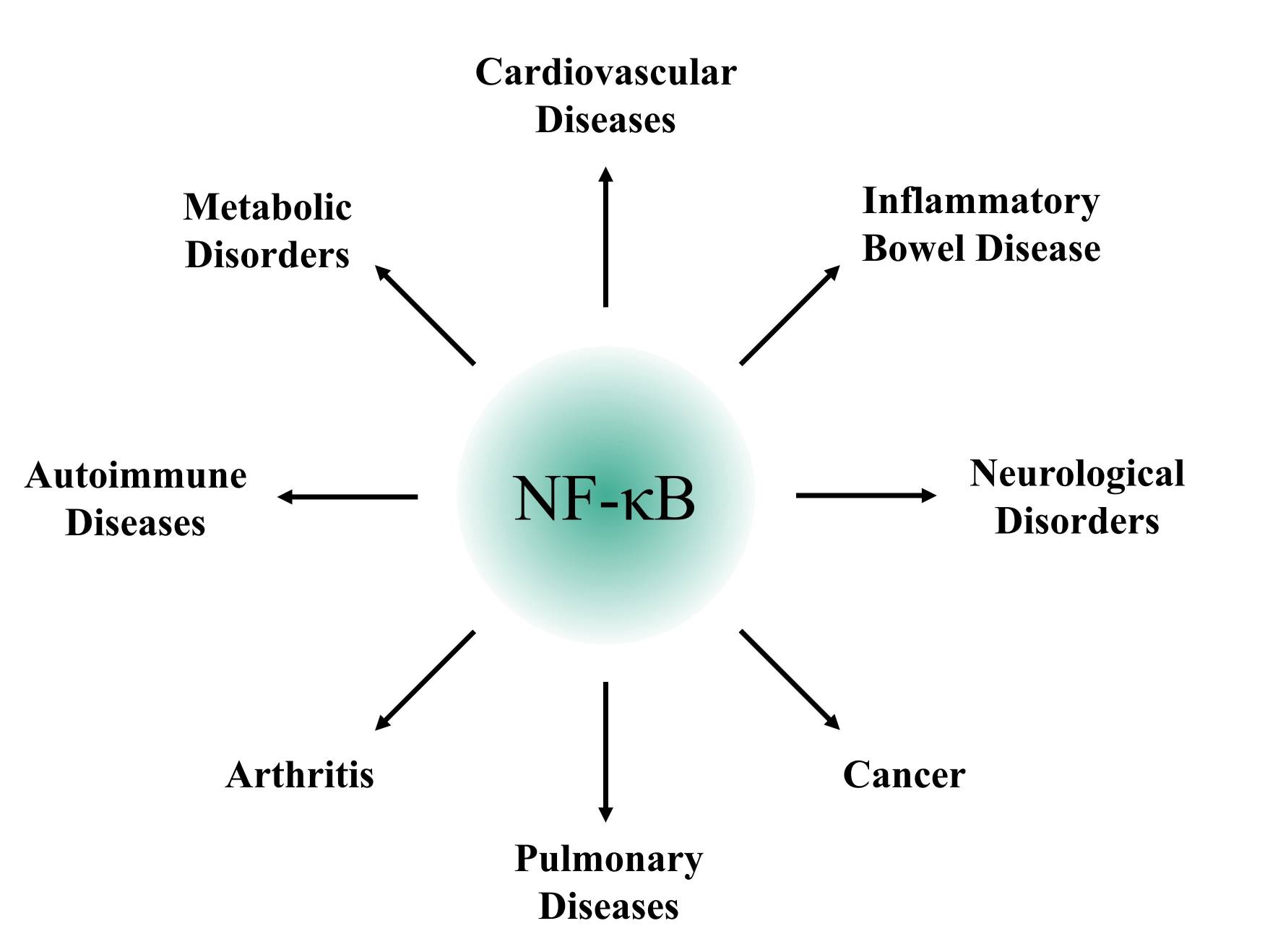 Fig. 5: NFκB associated diseases.
Fig. 5: NFκB associated diseases.NFκB has also been implicated in cancer initiation and progression. In many cancers, NFκB is constitutively active, driving the expression of genes that promote cell proliferation, inhibit apoptosis, and support angiogenesis and metastasis. For example, in hematological malignancies such as multiple myeloma and certain lymphomas, NFκB activation is often driven by mutations in upstream signaling components, leading to uncontrolled cell growth and resistance to apoptosis. Targeting the NFκB pathway is therefore an attractive therapeutic strategy in oncology, and several inhibitors have been developed to block NFκB signaling in cancer cells.
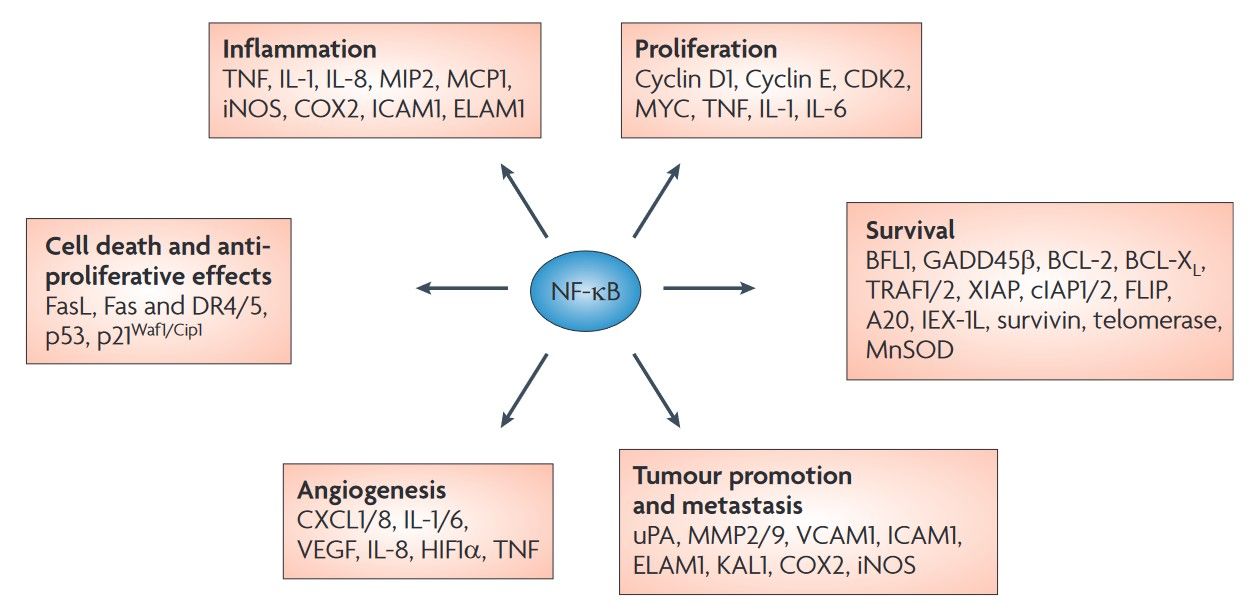 Fig. 6: NFκB target genes involved in cancer development and progression. Nuclear factor κB (NFκB) activation affects all six hallmarks of cancer through the transcription of genes involved in cell proliferation, angiogenesis, metastasis, inflammation and suppression of apoptosis as identified in both cell lines and tissue samples (Baud and Karin, 2009).
Fig. 6: NFκB target genes involved in cancer development and progression. Nuclear factor κB (NFκB) activation affects all six hallmarks of cancer through the transcription of genes involved in cell proliferation, angiogenesis, metastasis, inflammation and suppression of apoptosis as identified in both cell lines and tissue samples (Baud and Karin, 2009).Therapeutic Targeting of the NFκB Pathway
Given its central role in inflammation and cancer, the NFκB pathway is a major target for therapeutic intervention. Various strategies have been employed to inhibit NFκB activation, including the development of small-molecule inhibitors that target IKKβ, proteasome inhibitors that prevent IκB degradation, and inhibitors of upstream signaling kinases like NIK. Inhibitors of the NFκB pathway have shown promise in preclinical and clinical studies for the treatment of inflammatory diseases and certain cancers.
However, given the diverse and context-dependent roles of NFκB, therapeutic targeting must be approached with caution to avoid unintended immunosuppression or adverse effects on tissue homeostasis. Future research aims to develop more selective NFκB inhibitors that can modulate specific aspects of the pathway while preserving its essential physiological functions.
Case Study
Case 1: Kenneth, N. S.; et al. TfR1 interacts with the IKK complex and is involved in IKK–NFκB signalling. Biochemical Journal, 2013; 449(1), 275–284.
Transferrin receptor protein 1 (TfR1), also known as Cluster of Differentiation 71 (CD71), is a protein encoded by the TFRC gene in humans. TfR1 is required for the import of iron from transferrin into cells by endocytosis. In the context of the NFκB pathway, depletion of TfR1 does not lead to changes in IKK subunit protein levels; however, it reduces the formation of the IKK complex and inhibits TNFα (tumor necrosis factor α)-induced NFκB-dependent transcription. Kenneth and his colleagues found that TfR1 interacts with the IKK complex and is involved in IKK-NFκB signaling.
Activation of the canonical NFκB pathway by TNFα requires translocation of the RelA subunit from the cytoplasm to the nucleus, where it binds to target gene promoters and enhancers. To investigate the role of TfR1 in this process, they transfected U2OS cells with either control or TfR1 siRNA oligonucleotides, treated them with TNFα for varying durations, and then isolated nuclear fractions. In control cells, we observed a strong translocation of RelA to the nucleus within 30 minutes of TNFα stimulation. However, in cells depleted of TfR1, nuclear RelA levels were significantly reduced. Despite no change in total RelA levels, TfR1 depletion resulted in a decrease in RelA phosphorylation at Ser536, a site known to be phosphorylated by IKK. These results suggest that IKK function is impaired in the absence of TfR1.
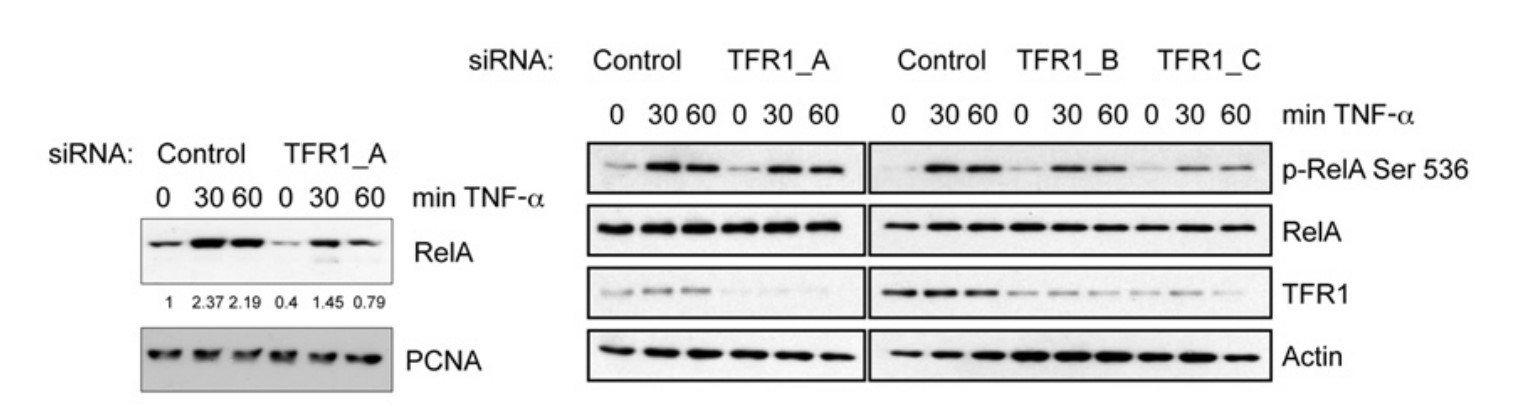 Fig. 7: Nuclear and cytoplasmic extracts were prepared from control cells or cells depleted of TfR1, and subsequently treated with TNFα for the times indicated. Nuclear extracts were analysed by Western blotting using the antibodies indicated. Whole-cell lysates were analysed for total levels of RelA under the same conditions. Levels of nuclear RelA were quantified and are shown below the RelA panel on the left.
Fig. 7: Nuclear and cytoplasmic extracts were prepared from control cells or cells depleted of TfR1, and subsequently treated with TNFα for the times indicated. Nuclear extracts were analysed by Western blotting using the antibodies indicated. Whole-cell lysates were analysed for total levels of RelA under the same conditions. Levels of nuclear RelA were quantified and are shown below the RelA panel on the left.Case 2: Lazarian, G.; et al. Stabilization of β-catenin upon B-cell receptor signaling promotes NF-kB target genes transcription in mantle cell lymphoma. Oncogene, 2020; 39(14), 2934–2947.
The B-cell receptor (BCR) is a transmembrane protein on the surface of a B cell that is activated by antigen binding. Activation of the BCR has two critical functions. One function is signal transduction, which involves changes in receptor oligomerization. The second function is to mediate internalization for subsequent processing of the antigen and presentation of peptides to helper T cells. The B cell receptor has been implicated in the pathogenesis of several B cell-derived lymphoid cancers.
Since both BCR and β-catenin are important mediators of cell survival and microenvironment interaction, the researchers examined the crosstalk between BCR and WNT/β-catenin signaling. They found that BCR stimulation stabilizes β-catenin, leading to increased levels of WNT16, an NFκB target gene, in mantle cell lymphoma (MCL) cells.
Primary MCL cells, Maver and Granta-519 cell lines, were stimulated with anti-IgM antibody for 1 hour and cells were subjected to cell fractionation. β-Catenin was detected in both cytosolic and nuclear fractions, and BCR stimulation resulted in β-Catenin stabilization in both fractions. Using confocal microscopy, the researchers confirmed nuclear accumulation of β-catenin upon BCR stimulation. While β-catenin staining was essentially cytoplasmic in unstimulated cells, BCR stimulation induced β-catenin translocation into the nucleus.
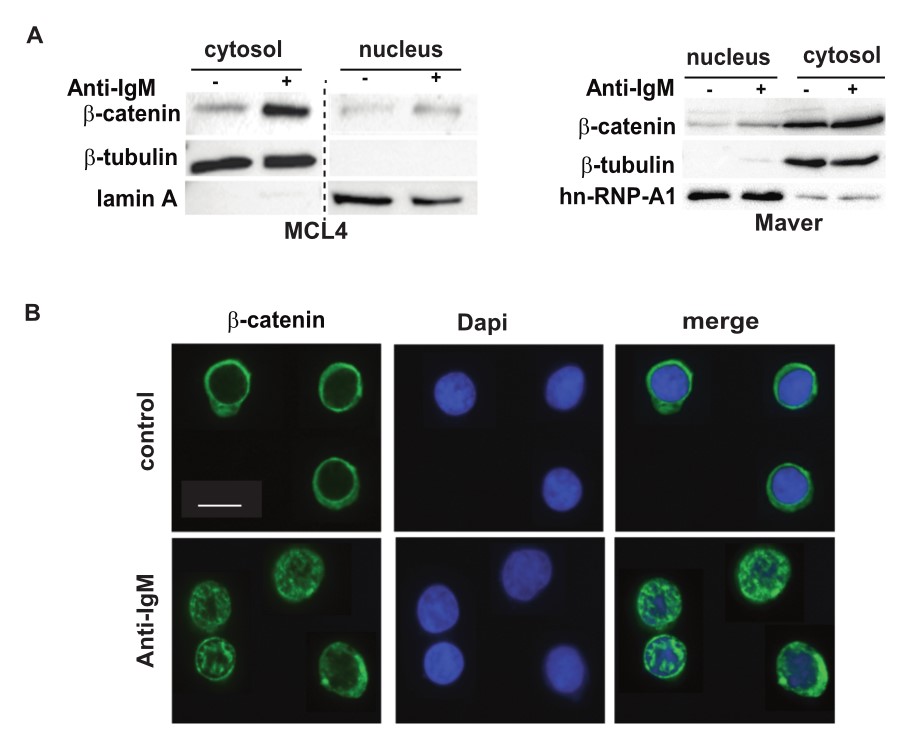 Fig. 8: BCR engagement induces β-catenin stabilization and nuclear translocation. A: Cell fractionation. Primary PBMCs (MCL4) and Maver cell line were stimulated (+) or not (-) with 10 µg/mL of soluble F(ab)'2 anti-IgM for 1 h. Cytosolic and nuclear fractions were prepared and analyzed by immunoblotting with the indicated Abs. β-tubulin and Lamin A were used to verify the purity of the cytoplasmic and nuclear fractions respectively. The figure is derived from the same gel and the dotted line represents the omitted lanes. The image is representative of two independent experiments. B: Immunofluorescence. Primary PBMCs (MCL9) were stimulated (F(ab)'2 anti IgM, 1 h, 10 µg/mL) or not (control). Confocal microscopy was performed using Alexa-488 coupled anti-β-catenin and DAPI to label nuclei. A minimum of 20 cells were analyzed. The scale bar corresponds to 10 µm.
Fig. 8: BCR engagement induces β-catenin stabilization and nuclear translocation. A: Cell fractionation. Primary PBMCs (MCL4) and Maver cell line were stimulated (+) or not (-) with 10 µg/mL of soluble F(ab)'2 anti-IgM for 1 h. Cytosolic and nuclear fractions were prepared and analyzed by immunoblotting with the indicated Abs. β-tubulin and Lamin A were used to verify the purity of the cytoplasmic and nuclear fractions respectively. The figure is derived from the same gel and the dotted line represents the omitted lanes. The image is representative of two independent experiments. B: Immunofluorescence. Primary PBMCs (MCL9) were stimulated (F(ab)'2 anti IgM, 1 h, 10 µg/mL) or not (control). Confocal microscopy was performed using Alexa-488 coupled anti-β-catenin and DAPI to label nuclei. A minimum of 20 cells were analyzed. The scale bar corresponds to 10 µm.References
- Baud, V., & Karin, M. (2009). Is NF-kappaB a good target for cancer therapy? Hopes and pitfalls. Nature Reviews. Drug Discovery, 8(1), 33–40.
- Guo, Q., Jin, Y., Chen, X., Ye, X., Shen, X., Lin, M., Zeng, C., Zhou, T., & Zhang, J. (2024). NFκB in biology and targeted therapy: New insights and translational implications. Signal Transduction and Targeted Therapy, 9(1), 1–37.
- Hayden, M. S., & Ghosh, S. (2008). Shared principles in NF-kappaB signaling. Cell, 132(3), 344–362.
- Kenneth, N. S., Mudie, S., Naron, S., & Rocha, S. (2013). TfR1 interacts with the IKK complex and is involved in IKK–NFκB signalling. Biochemical Journal, 449(1), 275–284.
- Lazarian, G., Friedrich, C., Quinquenel, A., Tran, J., Ouriemmi, S., Dondi, E., Martin, A., Mihoub, I., Chiron, D., Bellanger, C., Fleury, C., Gélébart, P., McCormack, E., Ledoux, D., Thieblemont, C., Marzec, J., Gribben, J. G., Cymbalista, F., Varin-Blank, N., … Baran-Marszak, F. (2020). Stabilization of β-catenin upon B-cell receptor signaling promotes NF-kB target genes transcription in mantle cell lymphoma. Oncogene, 39(14), 2934–2947.
- Liu, T., Zhang, L., Joo, D., & Sun, S.-C. (2017). NFκB signaling in inflammation. Signal Transduction and Targeted Therapy, 2(1), 1–9.
- Sun, S.-C. (2011). Non-canonical NFκB signaling pathway. Cell Research, 21(1), 71–85.

