CHD Family
Related Symbol Search List
Immunology Background
Background
CHD (Chromodomain Helicase DNA-binding) family proteins, also known as chromatin remodelers, play crucial roles in regulating gene expression by modifying the structure of chromatin.
Structure of CHD Family Proteins
CHD family proteins are characterized by the presence of two N-terminal chromodomains and a C-terminal SNF2-like ATPase domain.
| Domains | Details |
|---|---|
| Chromodomains (CHDs) |
Location: Typically, CHD proteins contain two N-terminal chromodomains. Function: These domains are responsible for recognizing specific histone modifications, such as methylated lysines, and are involved in targeting the protein to chromatin regions with these modifications. |
| SNF2-like ATPase Domain |
Location: Found at the C-terminal end of CHD proteins. Function: This domain has ATPase activity, providing the energy needed for chromatin remodeling by facilitating nucleosome sliding, positioning, or eviction. |
| Other Functional Domains | Some CHD proteins may contain additional domains, such as the SANT domain, PHD zinc finger domain, and BRK domain, which contribute to their specific functions and interactions within chromatin remodeling complexes. |
CHD proteins are mainly divided into three subfamilies by their specific domains, subfamily I with basic chromodomian and sucrose non-fermentable2 (SNF2)-like ATP-dependent helicase domain, subfamily II with additional PHD zinc finger domain, and subfamily III with additional SANT domain and BRK domain.
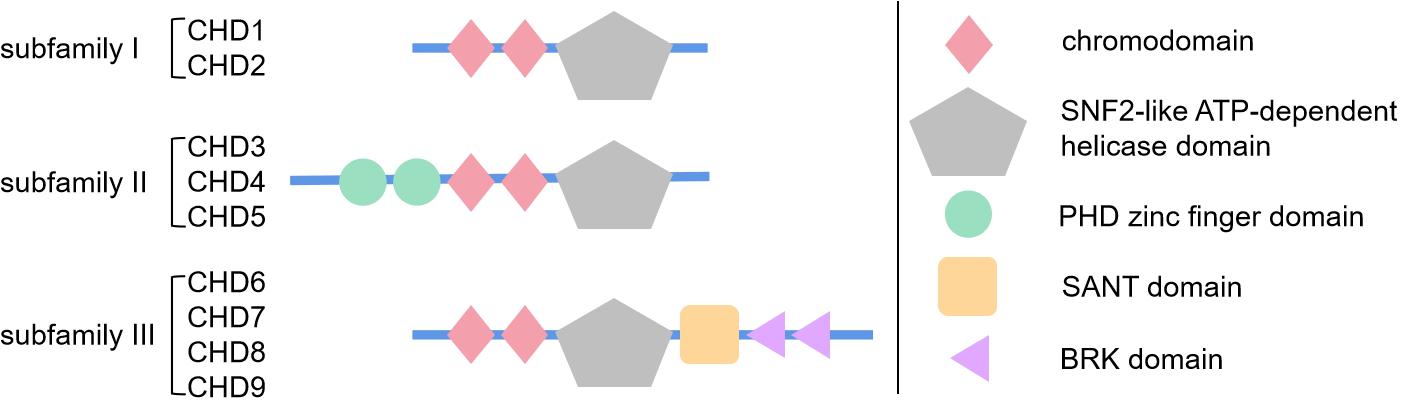 Fig.1 Schematic diagram showing the structure and specific domains of three subfamilies of chromodomain helicase DNA-binding (CHD) proteins. (Liu C, et al., 2021)
Fig.1 Schematic diagram showing the structure and specific domains of three subfamilies of chromodomain helicase DNA-binding (CHD) proteins. (Liu C, et al., 2021)Functions of CHD Family Proteins
Here are the key functions of CHD family proteins:
| Functions | Details |
|---|---|
| Chromatin Remodeling |
Nucleosome Dynamics: CHD proteins use ATP hydrolysis to alter nucleosome positioning, spacing, and composition, affecting the accessibility of DNA regions for transcriptional machinery. Transcriptional Regulation: By remodeling chromatin, CHD proteins can either activate or repress gene expression by controlling the accessibility of promoter and enhancer regions. |
| Gene Regulation |
Transcription Activation and Repression: CHD proteins participate in the regulation of gene expression by either promoting or inhibiting the recruitment of transcription factors to specific genomic regions. Enhancer Function: They are involved in regulating enhancer-promoter interactions and enhancer activity, influencing gene expression patterns. |
| Epigenetic Regulation |
Histone Modifications: CHD proteins can interact with and recognize specific histone modifications, contributing to the establishment and maintenance of epigenetic marks on chromatin. Chromatin Accessibility: They modulate chromatin accessibility, influencing the binding of transcription factors and other regulatory proteins to DNA. |
| DNA Repair and Replication |
DNA Damage Response: Some CHD family members are involved in DNA repair pathways, contributing to the maintenance of genomic integrity. Replication Dynamics: They play roles in ensuring proper chromatin structure during DNA replication, preventing errors, and maintaining genome stability. |
| Development and Differentiation |
Cell Fate Determination: CHD proteins are crucial for developmental processes, including cell fate determination, differentiation, and tissue-specific gene expression. Embryonic Development: They play essential roles in embryogenesis and organogenesis by regulating the expression of developmentally important genes. |
| Disease Associations | Dysregulation of CHD proteins has been linked to various diseases, including cancer, neurodevelopmental disorders, and developmental defects. Mutations in CHD genes can disrupt normal chromatin structure and gene expression, contributing to disease pathogenesis. |
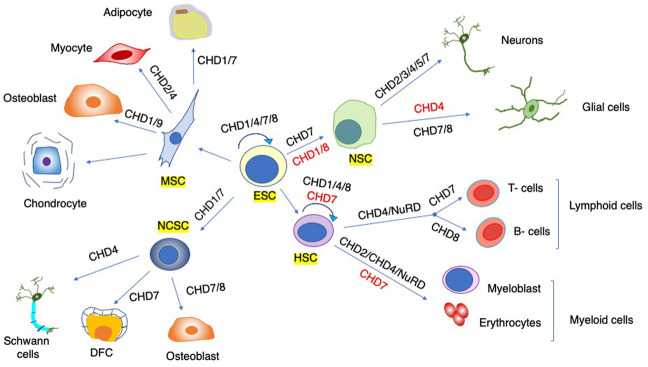 Fig.2 Cell fate specification and lineage commitment. (Alendar A, et al., 2021)
Fig.2 Cell fate specification and lineage commitment. (Alendar A, et al., 2021)
CHD functions are important in diverse stem cell populations (annotated by yellow rectangles). Some CHDs are important for the emergence and self-renewal of stem cells (circular arrows), and others are vital for lineage commitment and differentiation (straight arrows). Distinct CHDs can promote (black) or inhibit (red) differentiation. For simplicity, not all intermediate precursors and terminally differentiated cells are depicted. (ESC) Embryonic stem cell, (HSC) hematopoietic stem cell, (MSC) mesenchymal stem cell, (NSC) neural stem cell, (NCSC) neural crest stem cell, (DFC) dental follicle cells.
Members and Features of the CHD Family
Here are some key CHD family members and their features:
| Members | Features and function |
|---|---|
| CHD1 |
Features: Contains two N-terminal chromodomains and an ATPase domain. Function: Involved in transcriptional regulation, nucleosome positioning, and DNA repair. |
| CHD2 |
Features: Contains chromodomains and an SNF2-like ATPase domain. Function: Plays a role in chromatin remodeling, DNA repair, and transcriptional regulation. |
| CHD3 (Mi-2α) |
Features: Contains chromodomains and an ATPase domain. Function: Part of the NuRD (Nucleosome Remodeling and Deacetylase) complex, involved in transcriptional repression and chromatin remodeling. |
| CHD4 (Mi-2β) |
Features: Contains chromodomains, an ATPase domain, and a PHD zinc finger domain. Function: Component of the NuRD complex, that regulates gene expression, chromatin structure, and DNA repair. |
| CHD5 |
Features: Contains chromodomains, an ATPase domain, and a PHD zinc finger domain. Function: Implicated in neuronal development, tumor suppression, and epigenetic regulation. |
| CHD6 |
Features: Contains chromodomains, an ATPase domain, a SANT domain, and a BRK domain. Function: Involved in transcriptional regulation and chromatin remodeling processes. |
| CHD7 |
Features: Contains chromodomains, an ATPase domain, a SANT domain, and a BRK domain. Function: Critical for embryonic development, craniofacial patterning, and neural crest cell differentiation. |
| CHD8 |
Features: Contains chromodomains, an ATPase domain, a SANT domain, and a BRK domain. Function: Regulates gene expression, neural development, and autism-related pathways. |
The CHD family members exhibit both overlapping and distinct functions, contributing to their roles in chromatin dynamics, gene regulation, and cellular processes. Their unique features make them key players in the field of chromatin biology and epigenetic regulation.
The Impact of CHD Family on Disease Mechanisms
The CHD family of proteins plays a significant role in disease mechanisms, especially in conditions where chromatin structure and gene expression are dysregulated. Here is an overview of how the CHD family impacts disease mechanisms:
Cancer
- Tumor Suppression: Some CHD proteins, like CHD5, have been identified as tumor suppressors. Their loss or inactivation can lead to uncontrolled cell growth and tumorigenesis.
- Metastasis and Invasion: Dysregulation of CHD proteins can promote tumor metastasis and invasion by altering gene expression patterns related to cell adhesion, migration, and invasion.
- Epigenetic Alterations: Changes in the expression or activity of CHD family members can result in aberrant epigenetic modifications, contributing to cancer development and progression.
Neurodevelopmental Disorders
- Coffin-Siris Syndrome: Mutations in CHD7 are associated with Coffin-Siris syndrome, a rare genetic disorder characterized by developmental delays, intellectual disability, and distinctive physical features.
- Autism Spectrum Disorders (ASD): CHD8 mutations have been linked to autism spectrum disorders, affecting neural development and synaptic function.
- Neurological Impairments: Dysregulation of CHD family proteins can impact neuronal development, synaptic plasticity, and cognitive function, leading to various neurodevelopmental disorders.
Developmental Defects
- Embryonic Development: CHD proteins are critical for embryonic development and organogenesis. Mutations in CHD genes can disrupt normal development, leading to structural abnormalities and developmental defects.
- Craniofacial Abnormalities: CHD7 mutations are associated with craniofacial defects and developmental disorders like CHARGE syndrome.
Epigenetic Disorders
- Chromatin Dysregulation: Alterations in CHD protein expression or function can disrupt chromatin structure and gene expression profiles, contributing to epigenetic disorders.
- Imprinting Disorders: CHD family members are involved in regulating genomic imprinting, and their dysregulation can lead to imprinting disorders like Beckwith-Wiedemann syndrome.
Cardiovascular Diseases
- Heart Development: CHD proteins are essential for heart development and cardiac gene expression. Mutations in CHD genes can lead to congenital heart defects and cardiovascular diseases.
Immune Disorders
- Immune Response: CHD proteins play roles in regulating immune cell differentiation, function, and immune response gene expression. Dysregulation can contribute to immune disorders and autoimmune diseases.
Understanding the roles of CHD proteins in chromatin remodeling and gene regulation is crucial for elucidating the molecular mechanisms underlying these diseases and exploring potential therapeutic interventions.
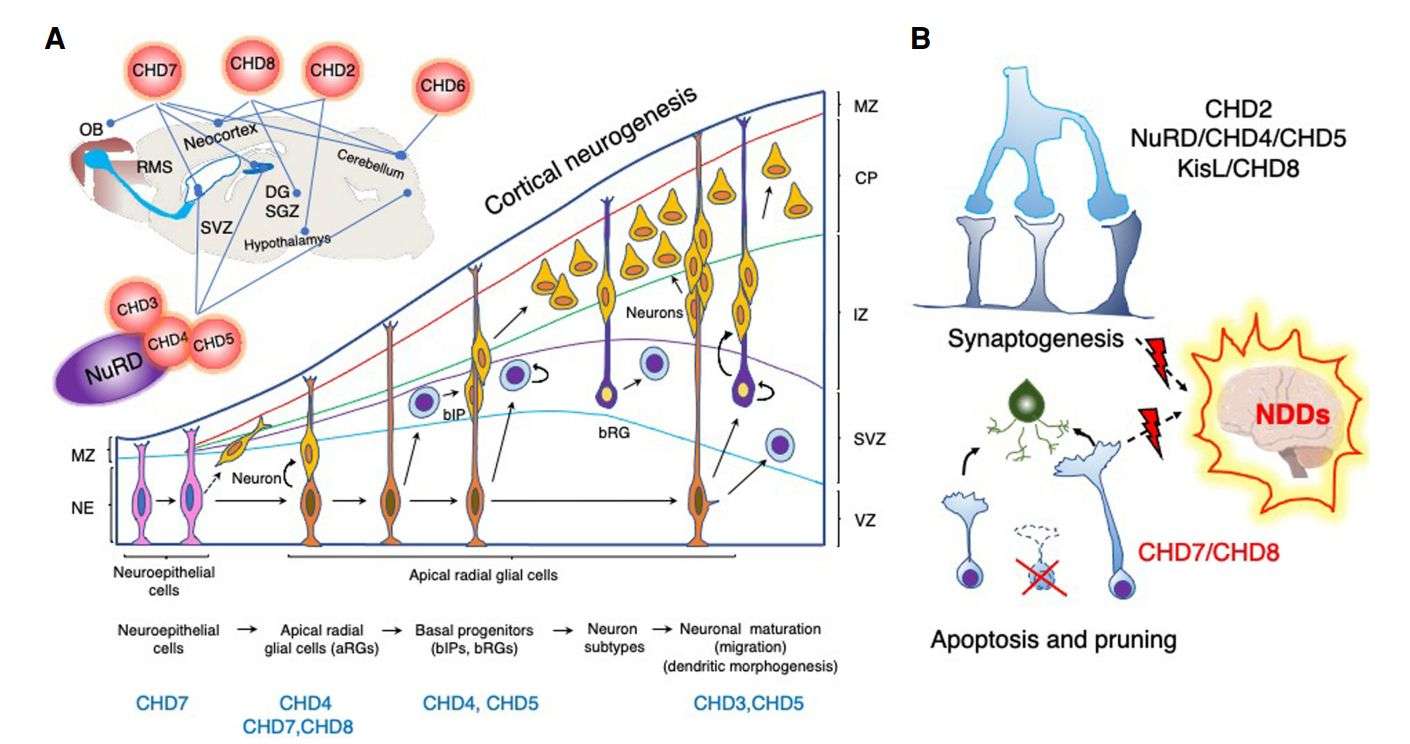 Fig.3 CHDs regulate different aspects of neurodevelopment. (Alendar A, et al., 2021)
Fig.3 CHDs regulate different aspects of neurodevelopment. (Alendar A, et al., 2021)
CHD enzymes are vital for the development and functionality of different parts of the brain: the cerebral neocortex (CHD2, CHD3/4/5/NuRD, CHD7, and CHD8), cerebellum (CHD4/NuRD, CHD6, CHD7, and CHD8), hypothalamus (CHD2), thalamus (CHD8), and hippocampus (CHD4/5/NuRD, CHD7, and CHD8). They regulate neural stem cell niches in the cerebrum (subventricular zone [SVZ]) and hippocampus (subgranular zone of the dentate gyrus [SGZ/DG]) and are essential for proper neuronal differentiation and migration. CHD7 is important for the development of the inner ear and olfactory system and regulates migration of the neuroblasts that emerge from the neural stem cells in the SVZ and proceed through the rostral migratory stream (RMS) to the olfactory bulb (OB). The cartoon represents the sagittal section of the rodent brain and highlights CHD functions in the respective brain regions (lines).
Case Study
Case 1: Moore S, Berger ND, Luijsterburg MS, et al. The CHD6 chromatin remodeler is an oxidative DNA damage response factor. Nat Commun. 2019;10(1):241.
Cell survival following oxidative DNA damage necessitates a coordinated interplay of signaling, repair, and transcriptional events, often facilitated by nucleosome dynamics mediated by chromatin remodeling enzymes. In this study, Chromodomain Helicase DNA-binding protein 6 (CHD6) is highlighted as a unique player in this process, distinct from other CHD enzymes. During oxidative stress, CHD6 is stabilized due to reduced degradation. It swiftly relocates to sites of DNA damage, responding to oxidative lesions through a poly(ADP-ribose)-dependent recruitment motif at its N-terminus, with subsequent anchoring facilitated by its double chromodomain and central core regions.
Depletion of CHD6 results in heightened persistence of reactive oxygen species, and impairs antioxidant transcriptional responses, leading to increased DNA damage and poly(ADP-ribose) induction. Notably, mutations in the catalytic domain or double chromodomain fail to rescue these effects. Despite no apparent epigenetic or DNA repair anomalies, loss of CHD6 compromises cell viability under prolonged oxidative stress conditions. This deficiency leads to aberrant chromatin relaxation, intensified DNA damage signaling, and hypersensitivity in cell cycle checkpoints. The findings propose that CHD6 plays a pivotal role as a regulator in the cellular response to oxidative DNA damage.
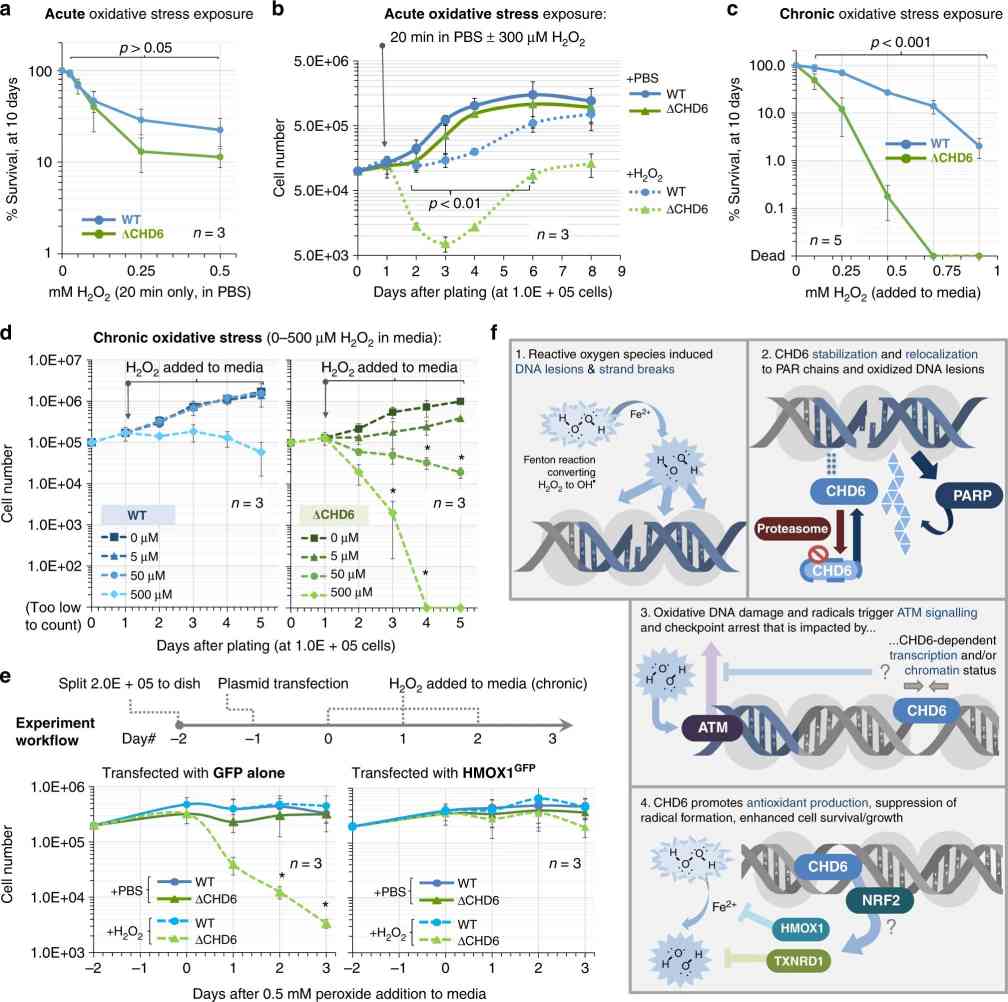 Fig.1 CHD6 loss causes failure to thrive after oxidative stress and perturbed oxidative base excision repair initiation.
Fig.1 CHD6 loss causes failure to thrive after oxidative stress and perturbed oxidative base excision repair initiation.Case 2: Jones KM, Sarić N, Russell JP, Andoniadou CL, Scambler PJ, Basson MA. CHD7 maintains neural stem cell quiescence and prevents premature stem cell depletion in the adult hippocampus. Stem Cells. 2015;33(1):196-210.
In the hippocampus, neural stem/progenitor cells (NSCs) continuously generate new neurons in adult life. These NSCs are typically kept in a state of reversible dormancy, and failure to maintain this dormant state can lead to premature depletion of the stem cell reservoir. The specific epigenetic mechanisms responsible for preserving this dormant state have remained unidentified. Through the use of a mouse model where CHD7, a chromatin remodeling factor, can be selectively deactivated, we demonstrate that CHD7 plays a critical role in preserving NSC dormancy.
Deactivation of CHD7 in adult NSCs within the hippocampus triggers a loss of dormancy among stem cells, prompting a temporary surge in cell divisions, which ultimately culminates in a notable decrease in neurogenesis. This loss of NSC dormancy correlates with an early decline in NSCs in aging mice. Our research reveals that CHD7 functions by suppressing the transcription of various genes that promote cell cycle progression and is essential for activating the Notch target gene Hes5 in dormant NSCs. These findings directly establish a connection between CHD7 and the pathways responsible for maintaining NSC dormancy, making it the first chromatin-remodeling factor identified with a role in this process.
Given that reduced CHD7 levels are associated with cognitive impairments seen in CHARGE syndrome, our discoveries may offer insights into the underlying causes of these deficits.
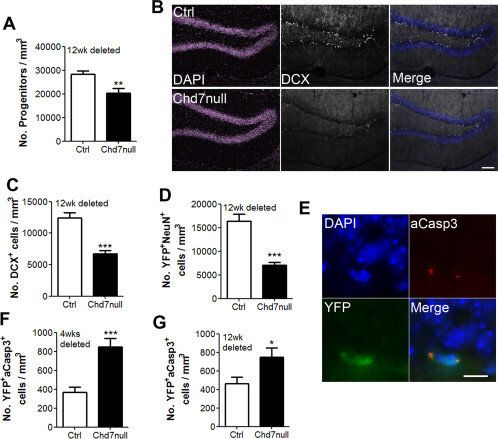 Fig.2 Loss of Chd7 results in a decrease in neurogenesis and apoptosis of newly generated cells.
Fig.2 Loss of Chd7 results in a decrease in neurogenesis and apoptosis of newly generated cells.Related References
- Liu C, Kang N, Guo Y, Gong P. Advances in chromodomain helicase DNA-binding (CHD) proteins regulating stem cell differentiation and human diseases. Front Cell Dev Biol. 2021;9:710203.
- Alendar A, Berns A. Sentinels of chromatin: chromodomain helicase DNA-binding proteins in development and disease. Genes Dev. 2021;35(21-22):1403-1430.
