Ferritin
-
Official Full Name
Ferritin -
Overview
This gene encodes the heavy subunit of ferritin, the major intracellular iron storage protein in prokaryotes and eukaryotes. It is composed of 24 subunits of the heavy and light ferritin chains. Variation in ferritin subunit composition may affect the rates of iron uptake and release in different tissues. A major function of ferritin is the storage of iron in a soluble and nontoxic state. Defects in ferritin proteins are associated with several neurodegenerative diseases. This gene has multiple pseudogenes. Several alternatively spliced transcript variants have been observed, but their biological validity has not been determined -
Synonyms
Human Liver Ferritin;Ferritin;apoferritin.
Recombinant Proteins
- Human
- Bovine
- Branchiostoma floridae
- Mouse
- Rat
- HEK293
- Bovine Spleen
- Human Spleen
- E.coli
- Human Heart
- Mouse Liver
- Rat Liver
- Mouse liver
- Non
- His
| Cat.# | Product name | Source (Host) | Species | Tag | Protein Length | Price |
|---|---|---|---|---|---|---|
| Ferritin-0088H | Recombinant Human Ferritin protein | HEK293 | Human |
|
||
| Ferritin-024B | Native Bovine Ferritin Protein, holo form | Bovine Spleen | Bovine | Non |
|
|
| Ferritin-025B | Native Bovine Ferritin Protein, apo-form | Bovine Spleen | Bovine | Non |
|
|
| Ferritin-026H | Native Human Ferritin Protein, holo form | Human Spleen | Human | Non |
|
|
| Ferritin-027H | Native Human Ferritin Protein, apo form | Human Spleen | Human | Non |
|
|
| Ferritin-1127B | Recombinant Branchiostoma floridae Ferritin Protein (Ala21-Leu234), N-His tagged | E.coli | Branchiostoma floridae | His | Ala21-Leu234 |
|
| Ferritin-179H | Native Human Ferritin | Human Heart | Human | Non |
|
|
| Ferritin-180M | Native Mouse Ferritin | Mouse Liver | Mouse | Non |
|
|
| Ferritin-181R | Native Rat Ferritin | Rat Liver | Rat | Non |
|
|
| Ferritin-20M | Native Mouse Ferritin protein | Mouse liver | Mouse | Non |
|
|
| Ferritin-36M | Mouse Ferritin Reference Standard | Mouse | Non |
|
||
| Ferritin-78R | Rat Ferritin Reference Standard | Rat | Non |
|
Background
What is ferritin protein?
Ferritin is found in almost all living organisms, including plants, animals and microbes. It acts as the major iron storage protein in animals. The main function of ferritin is to safely store iron intracellularly in a soluble and non-toxic form. It prevents iron overload and helps regulate free iron levels. Ferritin is made up of 24 subunits that self-assemble into a hollow spherical shell. The shell stores up to 4500 iron atoms within its cavity in the form of ferric oxyhydroxide mineral. The ferritin protein shell protects stored iron from participating in harmful redox reactions, while also making iron readily available for cell metabolic needs.
What is the function of ferritin protein?
Iron storage: Ferritin acts as the primary intracellular iron storage protein. It safely stores iron ions in its central cavity as insoluble ferric oxide mineral. This prevents iron-induced cellular toxicity.
Regulation of free iron levels: By sequestering and releasing iron in a controlled manner, ferritin regulates the levels of bioavailable "free" iron inside cells. This is important as excess free iron can lead to oxidative stress.
Protection from oxidative stress: By safely storing iron ions inside its cage-like structure, ferritin protects cells from iron-catalyzed reactive oxygen species (ROS) formation that can cause damage. This makes iron a less reactive metal.
Ferritin related signaling pathway
Iron regulatory protein (IRP)-iron responsive element (IRE) pathway: IREs in ferritin mRNA bind to IRPs under low iron conditions, inhibiting ferritin translation. When iron levels rise, IRP binding to IRE is reduced, releasing mRNA for ferritin synthesis.
Hypoxia inducible factor (HIF) pathway: Under hypoxia, HIF activates transcription of ferritin to sequester redox-active iron and reduce hydroxyl radical formation. Ferritin helps cells survive low oxygen stress.
PI3K/Akt/mTOR pathway: This induces ferritin synthesis by phosphorylating IRS-1 and activating transcription factors like NF-κB. It stimulates cell survival and plays a role in cancer.
NF-κB pathway: Inflammation triggers the NF-κB pathway which upregulates ferritin transcription. This prevents iron-mediated oxidative damage during immune responses.
FERRITIN Related Diseases
- Iron overload disorders - Hemochromatosis, transfusional iron overload have elevated ferritin due to excessive iron absorption/intake.
- Iron deficiency - Low ferritin indicates depleted iron stores as seen in iron deficiency anemia.
- Cancer - Increased ferritin is seen in several cancers and promotes tumor growth by regulating iron availability.
- Neurodegenerative diseases - Elevated ferritin is a marker for neurodegeneration in conditions like Alzheimer's and Parkinson's.
- Liver disease - Liver inflammation/damage leads to high ferritin levels indicating iron accumulation in tissues.
- Cancer therapy:Ferritin overexpression reduces iron availability for tumor growth, or can be used to selectively deliver anti-cancer drugs to tumors.
- Iron chelation therapy:Ferritin nanoparticles can encapsulate and transport iron-chelating drugs specifically to iron-overloaded tissues.
- Gene therapy:Viruses encoding ferritin are used to regulate iron levels in treatment of disorders like thalassemia and sickle cell disease.
- Cancer therapy:Ferritin overexpression reduces iron availability for tumor growth, or can be used to selectively deliver anti-cancer drugs to tumors.
- Iron chelation therapy:Ferritin nanoparticles can encapsulate and transport iron-chelating drugs specifically to iron-overloaded tissues.
- Gene therapy:Viruses encoding ferritin are used to regulate iron levels in treatment of disorders like thalassemia and sickle cell disease.
Case Study
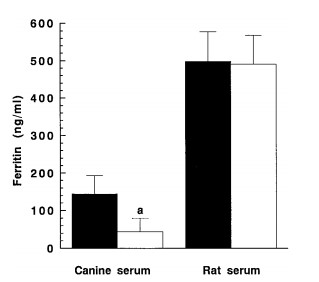
(Kiyotaka Watanabe, 1999)
Fig2. Effects of centrifugation on ferritin concentration in canine and rat sera. Canine and rat sera were centrifuged at 16, 000×g for 30 min at 4 ◦C, and ferritin in obtained supernatants (open bars) or in uncentrifuged sera (closed bars) was determined by ELISA. Each value is the mean ±SD of 12 dogs and 9 rats. a: Significantly different from the uncentrifuged canine sera .
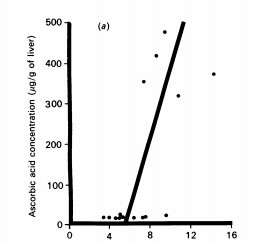
(Bill E. CHAM,, 1990)
Fig3. The iron/protein ratio expressed as a percentage is plotted against the concentration of ascorbic acid. The line drawn is based on least-squares linear-regression analysis. Correlation coefficients determined by linear-regression analyses of the plotted data were 0.7042 (P = 0.003) for CYF and 0.1064 (P = 0.717, not significant) for LAF.
Quality Guarantee
High Purity
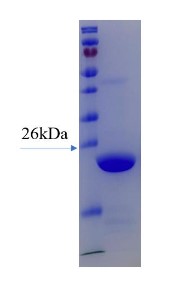
Fig1. SDS-PAGE (Cat. No.: Ferritin-0088H)
Involved Pathway
Ferritin involved in several pathways and played different roles in them. We selected most pathways Ferritin participated on our site, such as , which may be useful for your reference. Also, other proteins which involved in the same pathway with Ferritin were listed below. Creative BioMart supplied nearly all the proteins listed, you can search them on our site.
| Pathway Name | Pathway Related Protein |
|---|
Protein Function
Ferritin has several biochemical functions, for example, . Some of the functions are cooperated with other proteins, some of the functions could acted by Ferritin itself. We selected most functions Ferritin had, and list some proteins which have the same functions with Ferritin. You can find most of the proteins on our site.
| Function | Related Protein |
|---|
Interacting Protein
Ferritin has direct interactions with proteins and molecules. Those interactions were detected by several methods such as yeast two hybrid, co-IP, pull-down and so on. We selected proteins and molecules interacted with Ferritin here. Most of them are supplied by our site. Hope this information will be useful for your research of Ferritin.
Resources
Related Services
Related Products
References
- Polikarpov, D; Gabbasov, R; et al. Mossbauer study of exogenous iron redistribution between the brain and the liver after administration of (Fe3O4)-Fe-57 ferrofluid in the ventricle of the rat brain. JOURNAL OF MAGNETISM AND MAGNETIC MATERIALS 380:78-84(2015).
- Buck, A; Moore, LR; et al. Magnetic separation of algae genetically modified for increased intracellular iron uptake. JOURNAL OF MAGNETISM AND MAGNETIC MATERIALS 380:201-204(2015).


