EEF2
-
Official Full Name
eukaryotic translation elongation factor 2 -
Overview
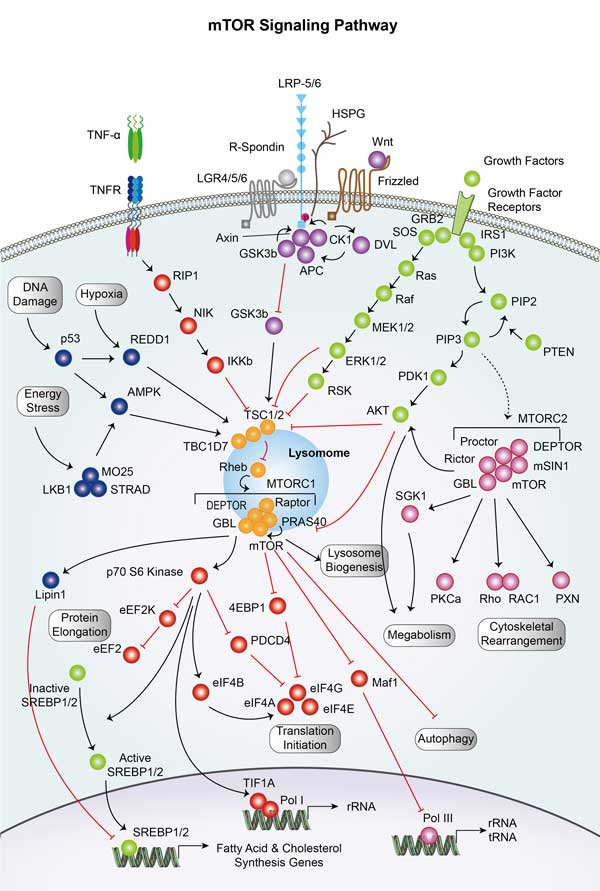
eEF2 (eukaryotic translation elongation factor 2) is essential for protein synthesis. It promotes the GTP-dependent translocation during peptide chain elongation. eEF2 is phosphorylated and inhibited by eEF2 kinase (eEF2K) on Thr56. eEF2K activity is inhibited by phosphorylation by both mTOR and p70S6K. This in turn up regulates eEF2 activity and protein synthesis. Similarly, AMPK down regulates protein synthesis by inhibited the activity of both p70S6K and mTOR, through TSC2. -
Synonyms
EEF2;eukaryotic translation elongation factor 2;EF2;elongation factor 2;EEF 2;polypeptidyl tRNA translocase;polypeptidyl-tRNA translocase;EF-2;EEF-2
Recombinant Proteins
- Human
- Rhesus macaque
- Rat
- Mouse
- Chicken
- Chinese hamster
- E.coli
- Mammalian Cells
- Wheat Germ
- HEK293
- Yeast
- In Vitro Cell Free System
- GST
- His
- DDK
- Myc
- Avi
- Fc
- Flag
Involved Pathway
EEF2 involved in several pathways and played different roles in them. We selected most pathways EEF2 participated on our site, such as AMPK signaling,AMPK signaling pathway,BDNF signaling pathway, which may be useful for your reference. Also, other proteins which involved in the same pathway with EEF2 were listed below. Creative BioMart supplied nearly all the proteins listed, you can search them on our site.
| Pathway Name | Pathway Related Protein |
|---|---|
| BDNF signaling pathway | JAK2,CDK5,SIRPA,YBX1,CFL1,CSNK2A1P,CSNK2A1,SYN1,LINGO1,FRS3 |
| Gene Expression | SNRNP25,KIAA1456,KHSRP,GLS2B,U2AF2,SMARCA5,ZNF124,AIMP2,CLP1,ZNF691 |
| Infectious disease | DOCK2,PDCD6IP,TAF1L,STX1B,SYT2,IPO5,VPS37A,ANTXR1,ANTXR2,CHMP4B |
| AMPK signaling | SLC2A4RG,LOC729991,ELAVL1,CAMKK2,INS-IGF2,CPT1B,CPT1A,ADRA1B,CDKN1A,ADRa1A |
| Gamma carboxylation, hypusine formation and arylsulfatase activation | DPH2,PROZ,GAS6,ARSJ,PROCA,ATPBD4,WDR85,ARSE,ARSH,EIF5A |
| Disease | RDBP,TLR10,STX1B,MYO18A,SUPT4H1,PACS1,TCEA1,RBP4,FGFR1OP,TNKS2 |
| Eukaryotic Translation Elongation | EEF1A1,EEF1D,EEF1G,EEF1A2,RPL35,EEF1B2 |
| AMPK signaling pathway | G6PC,GYS2,SCD5,PPP2R5B,RPS6KB1,CREB1,CREB3L3,LEP,PIK3R2,CREB3L2 |
Protein Function
EEF2 has several biochemical functions, for example, GTP binding,GTPase activity,poly(A) RNA binding. Some of the functions are cooperated with other proteins, some of the functions could acted by EEF2 itself. We selected most functions EEF2 had, and list some proteins which have the same functions with EEF2. You can find most of the proteins on our site.
| Function | Related Protein |
|---|---|
| translation elongation factor activity | EEF1G,TSFM,HBS1L,EEF1A1,EFTUD1,EEF2A.1,EEF1DA,EEF1A1L2,EEF1A1A,EEF1A1B |
| GTP binding | DNM1L,SAR1A,ARL4AA,MXB,EIF5B,RAB14L,HRAS,SEPT7,RAP2A,RAB12 |
| protein binding | HDAC7A,EIF2B4,TXN2,IMPA2,TIMM9,CAPRIN2,SCLY,LBR,NKX2-1,PIP5K1C |
| GTPase activity | ARL8A,EEF2A.2,RAB10,RHEB,RAB39A,RAB5AB,DNM2,LRRK2,GNAI2A,RAB40B |
| poly(A) RNA binding | CPEB2,RPS27A,DDX55,ADAR,ALG13,RPS18,HSP90AA1,PRPF6,RBM25,CHERP |
| protein kinase binding | AKAP1,PPP1CB,ACSL3,SPRED1,AP1B1,PARP1,CACNB3,FAF1,IL12RB2,ZBTB4 |
Interacting Protein
EEF2 has direct interactions with proteins and molecules. Those interactions were detected by several methods such as yeast two hybrid, co-IP, pull-down and so on. We selected proteins and molecules interacted with EEF2 here. Most of them are supplied by our site. Hope this information will be useful for your research of EEF2.
Akt2;RAD23A;MAX;YWHAZ;NDRG1;q81y32_bacan;MLH1;argD;sppA;glk1;q5nfm0_fratt;q7chc3_yerpe;trpE;q7cit1_yerpe;cysK;ytrA;q81qg7_bacan;ssuA
EEF2 Related Signal Pathway
Resources
Related Services
Related Products
References
- Grandjean, M; Sermeus, A; et al. Hypoxia Integration in the Serological Proteome Analysis Unmasks Tumor Antigens and Fosters the Identification of Anti-Phospho-eEF2 Antibodies as Potential Cancer Biomarkers. PLOS ONE 8:-(2013).
- Chen, CY; Fang, HY; et al. Sumoylation of eukaryotic elongation factor 2 is vital for protein stability and anti-apoptotic activity in lung adenocarcinoma cells. CANCER SCIENCE 102:1582-1589(2011).