STXBP1
-
Official Full Name
syntaxin binding protein 1 -
Overview
This gene encodes a syntaxin-binding protein. The encoded protein appears to play a role in release of;neurotransmitters via regulation of syntaxin, a transmembrane attachment protein receptor. Mutations in this gene have;been associated with infantile epileptic encephalopathy-4. Alternatively spliced transcript variants have been;described. -
Synonyms
STXBP1;syntaxin binding protein 1;syntaxin-binding protein 1;hUNC18;MUNC18 1;rbSec1;UNC18;N-Sec1;unc-18A;unc18-1;neuronal SEC1;protein unc-18 homolog 1;protein unc-18 homolog A;P67;NSEC1;MUNC18-1;FLJ37475
Recombinant Proteins
- Human
- Mouse
- Rhesus macaque
- Rat
- Chicken
- E.coli
- Mammalian Cell
- Wheat Germ
- Insect Cells
- Insect Cell
- HEK293
- HEK293T
- Mammalian cells
- GST
- His
- Non
- His&GST
- His&Fc&Avi
- Myc&DDK
- His&MBP
- Flag
Background
What is STXBP1 protein?
STXBP1 gene (syntaxin binding protein 1) is a protein coding gene which situated on the long arm of chromosome 9 at locus 9q34. STXBP1 is a gene that encodes the Munc18-1 protein, which is crucial for synaptic vesicle exocytosis and neurotransmitter release at nerve terminals. Mutations in the STXBP1 gene are associated with a range of clinical phenotypes, including developmental delays, intellectual disability, and various forms of epilepsy such as infantile spasms and Ohtahara syndrome. The protein plays a role in the regulation of synaptic transmission and is important for normal neuronal function and development. The STXBP1 protein is consisted of 594 amino acids and STXBP1 molecular weight is approximately 67.6 kDa.
What is the function of STXBP1 protein?
The STXBP1 protein, also known as Munc18-1, is an essential presynaptic protein that regulates synaptic vesicle exocytosis, a process critical for neurotransmitter release at nerve terminals. Mutations in the STXBP1 gene can lead to a spectrum of neurodevelopmental disorders, including epilepsy and intellectual disability. The protein plays a role in several cellular processes such as exocytosis, neuronal viability, and cellular transport.
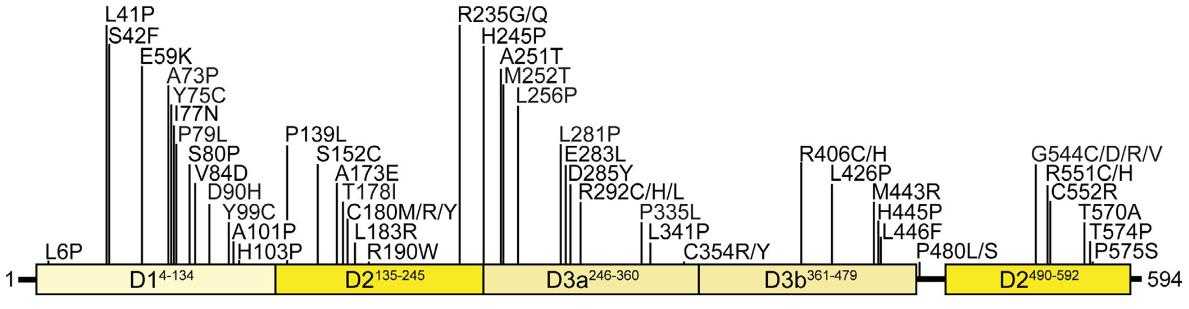
Fig1. Primary sequence of STXBP1 with indication of its domain structure and positions of disease-linked missense mutations. (Debra Abramov, 2021)
STXBP1 related signaling pathway
The STXBP1-related signaling pathway is intricately involved in the regulation of neurotransmitter release at synapses. STXBP1, also known as munc18-1, interacts with syntaxin-1 to facilitate the assembly of the SNARE complex, which is crucial for vesicle priming and subsequent exocytosis. This process is essential for proper neuronal communication and function. Dysregulation of this pathway can lead to impaired synaptic transmission and has been implicated in various neurodevelopmental and neurodegenerative disorders, underscoring its critical role in maintaining normal neural activity.
STXBP1 related diseases
Mutations in the STXBP1 gene are associated with a range of neurodevelopmental disorders, most notably early-onset epileptic encephalopathies like Ohtahara syndrome and West syndrome, as well as other developmental epileptic encephalopathies. These conditions are characterized by severe intellectual disability, refractory seizures, and often poor developmental outcomes. Additionally, STXBP1 mutations can present as non-syndromic epilepsies, atypical Rett syndrome, and severe intellectual disability without epilepsy. The phenotypic spectrum of STXBP1-related disorders is broad, but all patients have some form of intellectual disability, and the majority experience epilepsy, often from an early age.
Bioapplications of STXBP1
The bioapplications of STXBP1 are predominantly focused on its pivotal role in the regulation of neurotransmitter release at synapses. As a key component of the vesicle priming and exocytosis machinery, STXBP1 (also known as munc18-1) interacts with syntaxin-1 to facilitate the assembly of the SNARE complex, which is essential for proper neuronal communication and function. Research into STXBP1 holds significant promise for understanding and treating various neurodevelopmental and neurodegenerative disorders characterized by impaired synaptic transmission. By elucidating the mechanisms underlying STXBP1's function, scientists aim to develop targeted therapies that can restore or enhance neural activity, thereby improving patient outcomes and quality of life.
Case Study
Case Study 1: Faye McLeod, 2023
Early infantile developmental and epileptic encephalopathies, often genetic, are challenging to study due to the difficulty of examining human cortical brain development during key prenatal periods. To overcome this, researchers developed an in vitro assay using organotypic cultures of human subplate and cortical regions from 14-17 week-old fetal brain tissue. This allowed to maintain and observe the development and synaptic network formation of neurons over several months. They applied this assay to study STXBP1 haploinsufficiency, a common genetic cause of these encephalopathies, and found it impaired synaptic function and reduced glutamatergic synapse density, demonstrating a method to assess genetic impacts on human cortical development.

Fig1. STXBP1 and NeuN (all neurons) immunofluorescence reveals location of the SP.
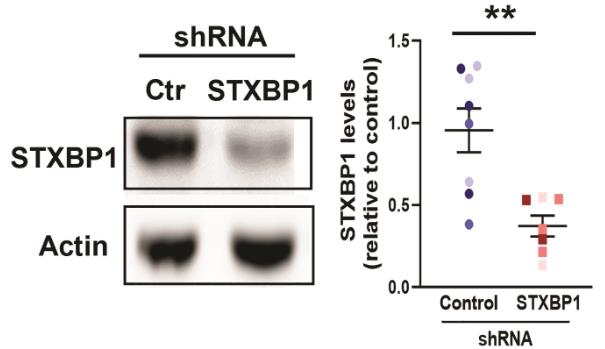
Fig2. A western blot showing knock-down of STXBP1 protein in a whole slice.
Case Study 2: Jovana Kovacevic, 2018
De novo heterozygous mutations in the STXBP1/Munc18-1 gene lead to early infantile epileptic encephalopathies, characterized by epilepsy, developmental delays, intellectual disability, and potential autism spectrum features. Researchers investigated the impact of seven STXBP1 mutations and created four mouse models to mimic the EEG abnormalities and cognitive deficits found in STXBP1-encephalopathy. The disease-causing variants affected synaptic transmission when absent but not when overexpressed, with all showing reduced protein levels, indicating protein instability and haploinsufficiency as key factors. Stxbp1+/- mice displayed seizure-like behaviors that responded to levetiracetam, and those with GABAergic neuron-specific deficiency showed increased mortality and seizure activity. These mice also exhibited cognitive impairments and anxiety-like behaviors. The models suggest that protein instability, haploinsufficiency, and cortical excitation imbalance are the core mechanisms of STXBP1-encephalopathy, providing a basis for potential therapeutic development.

Fig3. Dissociated cortical neurons were stained for Munc18-1 and synaptic marker synaptobrevin.

Fig4. Western blot analysis of Munc18-1 protein levels in Stxbp1 null neurons.
Quality Guarantee
High Purity
.jpg)
Fig1. SDS-PAGE (STXBP1-3600H)
Involved Pathway
STXBP1 involved in several pathways and played different roles in them. We selected most pathways STXBP1 participated on our site, such as Synaptic vesicle cycle, which may be useful for your reference. Also, other proteins which involved in the same pathway with STXBP1 were listed below. Creative BioMart supplied nearly all the proteins listed, you can search them on our site.
| Pathway Name | Pathway Related Protein |
|---|---|
| Synaptic vesicle cycle | ATP6V1B2,ATP6V1G2,CPLX4,UNC13C,ATP6V1A,ATP6V0D1,SYT1,ATP6V0A2,STX1B,ATP6V1E1 |
Protein Function
STXBP1 has several biochemical functions, for example, SNARE binding,identical protein binding,poly(A) RNA binding. Some of the functions are cooperated with other proteins, some of the functions could acted by STXBP1 itself. We selected most functions STXBP1 had, and list some proteins which have the same functions with STXBP1. You can find most of the proteins on our site.
| Function | Related Protein |
|---|---|
| syntaxin-1 binding | CPLX1,CCDC167,STXBP3,NSF,GOLSYN,SYT1,RNF40,LRRK2,SNPH,STXBP3A |
| poly(A) RNA binding | HNRNPH3,BICC1,CDK13,CPSF4,RBM25,RPL19,KIAA1429,MRPL4,FYTTD1,RTF1 |
| protein domain specific binding | GABRR2,RCC2,VAPA,IPCEF1,ZFP521,MYO1D,TBL1X,SKI,TACC2,NR0B2 |
| identical protein binding | S100A4,BCHE,CD28,RUNX1T1,BNIP3,HOMER1,GOT1,NQO1,RASSF1,SERPINA1E |
| syntaxin binding | SEC22B,SYT1B,VPS11,VPS54,NAPB,TXLNA,SYT13,STX6,SYT1A,SYT5A |
| SNARE binding | VTI1A,STX11A,CAPN10,TNFAIP2A,SEC22C,VAMP3,STX11B.1,VAMP2,EXOC3L,SNAPIN |
| protein N-terminus binding | ALG2,GLRX,APTX,ERCC2,TAF11,SMARCE1,SLC6A3,NFE2,RPA2,SUV39H1 |
| protein kinase binding | SYN1,FER,PRKAG1,PLK1,CALM3,SQSTM1,CDC25A,FOXO3,BCL2L14,CCNT2B |
| protein binding | ARAF,ZNF259,MYO1C,KLF17,ELP3,DOK4,BMP1A,SEC13,SPRED1,USH1C |
Interacting Protein
STXBP1 has direct interactions with proteins and molecules. Those interactions were detected by several methods such as yeast two hybrid, co-IP, pull-down and so on. We selected proteins and molecules interacted with STXBP1 here. Most of them are supplied by our site. Hope this information will be useful for your research of STXBP1.
TRIM38;STX11;STX19;PRKAR1A;PLD1;APP;ID1;HTT;Plk2;IQCB1;FERMT3;Phospho1;Sart3;DNAJC11
Resources
Related Services
Related Products
References
- Rodriguez, AR; Muller, LPD; et al. The RNA binding protein RBPMS is a selective marker of ganglion cells in the mammalian retina. JOURNAL OF COMPARATIVE NEUROLOGY 522:1411-1443(2014).
- Ramakrishnan, NA; Drescher, MJ; et al. Calcium Regulates Molecular Interactions of Otoferlin with Soluble NSF Attachment Protein Receptor ( SNARE) Proteins Required for Hair Cell Exocytosis*. JOURNAL OF BIOLOGICAL CHEMISTRY 289:8750-8766(2014).


