RAB38
-
Official Full Name
RAB38, member RAS oncogene family -
Overview
Rab38 is a member of the Rab small G protein family and is mainly expressed in lung alveolar type II cells and melanocytes. In mice, the G146T Rab38 gene mutation results in loss of Rab38 function and causes abnormal pigmentation due to the loss of melano -
Synonyms
RAB38;RAB38, member RAS oncogene family;ras-related protein Rab-38;NY MEL 1;melanoma antigen NY-MEL-1;Rab-related GTP-binding protein;rrGTPbp;NY-MEL-1
Recombinant Proteins
- Human
- Mouse
- E.coli
- HEK293
- HEK293T
- Mammalian cells
- GST
- His
- Non
- Myc&DDK
- His&Fc&Avi
- Flag
| Cat.# | Product name | Source (Host) | Species | Tag | Protein Length | Price |
|---|---|---|---|---|---|---|
| RAB38-2117H | Recombinant Human RAB38, GST-tagged | E.coli | Human | GST | 1-211aa | |
| RAB38-2727H | Recombinant Human RAB38 protein, His-tagged | E.coli | Human | His | 1-211 aa | |
| RAB38-2600HCL | Recombinant Human RAB38 293 Cell Lysate | HEK293 | Human | Non |
|
|
| RAB38-1089H | Recombinant Human RAB38 Protein, Myc/DDK-tagged, C13 and N15-labeled | HEK293T | Human | Myc&DDK |
|
|
| RAB38-1834H | Recombinant Human RAB38 Protein, His (Fc)-Avi-tagged | HEK293 | Human | His&Fc&Avi |
|
|
| RAB38-1834H-B | Recombinant Human RAB38 Protein Pre-coupled Magnetic Beads | HEK293 | Human |
|
||
| RAB38-2076HFL | Recombinant Full Length Human RAB38 Protein, C-Flag-tagged | Mammalian cells | Human | Flag | Full L. |
|
| Rab38-5314M | Recombinant Mouse Rab38 Protein, Myc/DDK-tagged | HEK293T | Mouse | Myc&DDK |
|
Background
What is RAB38 protein?
RAB38 (RAB38, member RAS oncogene family) gene is a protein coding gene which situated on the long arm of chromosome 11 at locus 11q14. Enables several functions, including AP-1 adaptor complex binding activity; AP-3 adaptor complex binding activity; and BLOC-2 complex binding activity. Involved in several processes, including endosome to melanosome transport; melanosome assembly; and phagosome acidification. Located in several cellular components, including cytoplasmic vesicle; lysosome; and mitochondria-associated endoplasmic reticulum membrane. RAB38 protein is consisted of 211 amino acids and its molecular mass is approximately 23.7 kDa.
What is the function of RAB38 protein?
The RAB38 protein may be involved in the transport and docking process of melanosomes, which is crucial for the synthesis and distribution of melanosomes. RAB38 plays a role in the maturation of phagosomes, particularly those involving phagocytic pathogens such as Staphylococcus aureus and Mycobacterium tuberculosis. RAB38 plays an important role in controlling melanin production and melanosome biosynthesis. Together with RAB32, RAB38 regulates the proper transport of melanin-producing enzymes such as tyrosinase (TYR), TYRP1, and DCT/TYRP2 to melanosomes in melanocytes. In glioblastoma cells, RAB38 helps with energy metabolism and fights cell death.
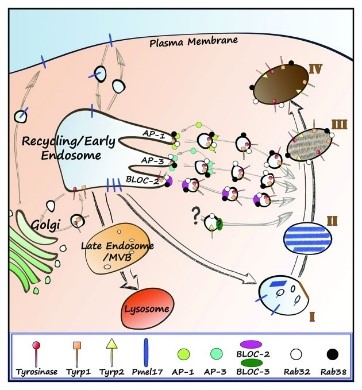
Fig1. Model of melanosome biogenesis. (Jarred J Bultema, 2013)
RAB38 Related Signaling Pathway
RAB38 protein may be involved in the transport and docking of melanosomes. Melanosomes are organelles responsible for the synthesis, storage and transport of melanin, and RAB38 plays an important role in this process. RAB38 is involved in the correct classification of TYRP1 (tyrosinase-associated protein 1), which is critical for the peripheral melanosome distribution of TYRP1 in melanocytes. Together with RAB32, RAB38 regulates the proper transport of melanin-producing enzymes such as tyrosinase (TYR), TYRP1, and DCT/TYRP2 to melanosomes in melanocytes. RAB38 regulates its phosphorylation of Rab10 through its interaction with LRRK2 (leucine-enriched repeat kinase 2), which may affect intracellular protein transport and cell membrane dynamics.
RAB38 Related Diseases
RAB38 protein is associated with a variety of diseases, and it is responsible for regulating vesicle transport and substance transport in the cell, so it is closely related to a variety of biological processes in the cell. Abnormal expression or dysfunction of RAB38 has been associated with the development of tumors, neurodegenerative diseases, immune-related diseases, and some genetic diseases. Specifically, RAB38 is associated with tumor recurrence and prognosis in non-small cell lung cancer and may play a role in tumor aggressiveness and metastasis. In addition, RAB38 promotes tumor growth in glioblastoma by affecting energy metabolism and cell survival. RAB38 is also associated with Crohn's disease, inflammatory bowel disease, late-onset Alzheimer's disease, frontotemporal dementia and other diseases. Abnormalities of RAB38 have also been observed in ocular skin albinism and retinal pigment epithelopathy.
Bioapplications of RAB38
In the field of drug discovery, the recombinant RAB38 protein can be used to screen and evaluate the effects of potential drugs on tumor cell proliferation and survival, especially in the study of non-small cell lung cancer and glioblastoma therapy. In the treatment of diseases, the RAB38 protein is also used in the development of therapeutic drugs for the treatment of cancer, autoimmune diseases, cardiovascular diseases, etc.
Case Study
Case Study 1: Bao-Yu Li, 2019
Ras‑Related Protein Rab‑38 (RAB38), which belongs to the RAB family, is involved in the biogenesis of lysosome‑related organelles and defense against certain microbial infections. However, the clinical significance and potential function of RAB38 in pancreatic adenocarcinoma remain unclear. To validate the role of RAB38 in tumors, the effect of RAB38 on tumor cell proliferation, migration and invasion was assessed by establishing RAB38 knockdown cell lines. Reverse transcription‑quantitative polymerase chain reaction and western blotting were used to examine the expression levels of proteins associated with the cancer cell behavior. In addition, the inhibitory effect of RAB38 silencing on pancreatic cancer was examined in mice. The immunohistochemistry results revealed that RAB38 was upregulated and positively correlated with the grade of progression in pancreatic adenocarcinoma patients. Further investigation indicated that RAB38 downregulation significantly suppressed the proliferation, migration and invasive capacity of pancreatic cancer cells, as well as decreased the expression levels of Ki67, proliferating cell nuclear antigen, and matrix metalloproteinases 2 and 9. RAB38 silencing also inhibited the development of pancreatic cancer in vivo.

Fig1. Cell viability in the RAB38 shRNA and control groups was analyzed using an MTT assay.
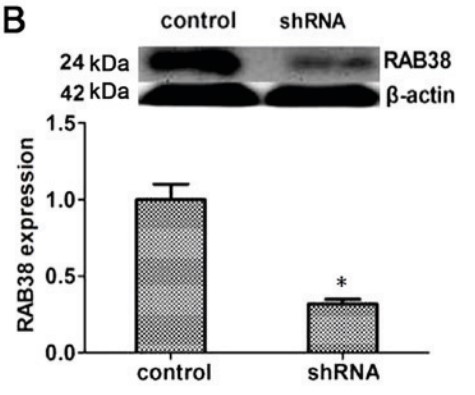
Fig2. Western blot analysis results of RAB38 expression in the two different groups.
Case Study 2: Elena Bianchetti, 2021
Glioblastoma is a high-grade glial neoplasm with a patient survival of 12-18 months. Therefore, the identification of novel therapeutic targets is an urgent need. RAB38 is a GTPase protein implicated in regulating cell proliferation and survival in tumors. The role of RAB38 in glioblastoma is relatively unexplored. Here, the researchers test the hypothesis that RAB38 regulates glioblastoma growth using human glioblastoma cell lines. They found that genetic interference of RAB38 resulted in a decrease in glioblastoma growth through inhibition of proliferation and cell death induction. Transcriptome analysis showed that RAB38 silencing leads to changes in genes related to mitochondrial metabolism and intrinsic apoptosis (e.g., Bcl-xL). Consistently, rescue experiments demonstrated that loss of RAB38 causes a reduction in glioblastoma viability through downregulation of Bcl-xL. Moreover, RAB38 knockdown inhibited both glycolysis and oxidative phosphorylation. Interference with RAB38 enhanced cell death induced by BH3-mimetics. RAB38 antagonists are under development, but not yet clinically available.

Fig3. Astrocytes were examined by Western blot analysis for RAB38 expression.

Fig4. LN229 glioblastoma cells overexpressing Bcl-xL stained with annexin V/propidium iodide.
Quality Guarantee
High Purity
.jpg)
Fig1. SDS-PAGE (RAB38-2076HFL)
Involved Pathway
RAB38 involved in several pathways and played different roles in them. We selected most pathways RAB38 participated on our site, such as , which may be useful for your reference. Also, other proteins which involved in the same pathway with RAB38 were listed below. Creative BioMart supplied nearly all the proteins listed, you can search them on our site.
| Pathway Name | Pathway Related Protein |
|---|
Protein Function
RAB38 has several biochemical functions, for example, AP-1 adaptor complex binding,NOT AP-2 adaptor complex binding,AP-3 adaptor complex binding. Some of the functions are cooperated with other proteins, some of the functions could acted by RAB38 itself. We selected most functions RAB38 had, and list some proteins which have the same functions with RAB38. You can find most of the proteins on our site.
| Function | Related Protein |
|---|---|
| AP-3 adaptor complex binding | PI4K2A,RAB32 |
| protein binding | EME1,CARKD,SOX17,LRRIQ3,HNF4A,HMGCR,CACNA2D1A,FAM9A,SCARA5,PPP2R2B |
| BLOC-2 complex binding | RAB32 |
| NOT AP-2 adaptor complex binding | RAB32 |
| GTP-dependent protein binding | AP1G1,RAPGEF5,CDC42,RAB32,GCH1,RAB5B,RASGRP4,GCHFR,EEA1,MRAS |
| AP-1 adaptor complex binding | RAB32,AP1AR,ARPC2 |
| GTPase activity | RHOT1A,IRGE4,RAB17,GTPBP4,RAB1BB,RIT2,RAB36,RAB35B,LSG1,GNAQ |
| GTP binding | RAB33BA,RAB21,HRAS,GUCY1A3,ACSM1,RHOBTB2A,RALBB,INSR,GUCY2C,GNAQ |
Interacting Protein
RAB38 has direct interactions with proteins and molecules. Those interactions were detected by several methods such as yeast two hybrid, co-IP, pull-down and so on. We selected proteins and molecules interacted with RAB38 here. Most of them are supplied by our site. Hope this information will be useful for your research of RAB38.
gtp
Resources
Related Services
Related Products
References
- Germain, C; Gnjatic, S; et al. Presence of B Cells in Tertiary Lymphoid Structures Is Associated with a Protective Immunity in Patients with Lung Cancer. AMERICAN JOURNAL OF RESPIRATORY AND CRITICAL CARE MEDICINE 189:832-844(2014).
- Biernacki, MA; Marina, O; et al. Efficacious Immune Therapy in Chronic Myelogenous Leukemia (CML) Recognizes Antigens That Are Expressed on CML Progenitor Cells. CANCER RESEARCH 70:906-915(2010).


