DYSF
-
Official Full Name
dysferlin -
Overview
The protein encoded by this gene belongs to the ferlin family and is a skeletal muscle protein found associated with the sarcolemma. It is involved in muscle contraction and contains C2 domains that play a role in calcium-mediated membrane fusion events, suggesting that it may be involved in membrane regeneration and repair. In addition, the protein encoded by this gene binds caveolin-3, a skeletal muscle membrane protein which is important in the formation of caveolae. Specific mutations in this gene have been shown to cause autosomal recessive limb girdle muscular dystrophy type 2B (LGMD2B) as well as Miyoshi myopathy. Alternative splicing results in multiple transcript variants. [provided by RefSeq, Aug 2008] -
Synonyms
DYSF;dysferlin;MMD1;FER1L1;LGMD2B;fer-1-like protein 1;dystrophy-associated fer-1-like 1;dystrophy-associated fer-1-like protein;limb girdle muscular dystrophy 2B (autosomal recessive);dysferlin, limb girdle muscular dystrophy 2B (autosomal recessive)
Recombinant Proteins
- Zebrafish
- Mouse
- Human
- Mammalian Cell
- HEK293T
- HEK293
- Mammalian cells
- E.coli
- His
- Myc&DDK
- Flag
- His&Fc&Avi
| Cat.# | Product name | Source (Host) | Species | Tag | Protein Length | Price |
|---|---|---|---|---|---|---|
| DYSF-8213Z | Recombinant Zebrafish DYSF | Mammalian Cell | Zebrafish | His |
|
|
| Dysf-2703M | Recombinant Mouse Dysf Protein, Myc/DDK-tagged | HEK293T | Mouse | Myc&DDK |
|
|
| DYSF-2773H | Recombinant Human DYSF Protein, MYC/DDK-tagged | HEK293 | Human | Myc&DDK |
|
|
| DYSF-4000H | Recombinant Human DYSF Protein, Myc/DDK-tagged, C13 and N15-labeled | HEK293T | Human | Myc&DDK |
|
|
| DYSF-518HFL | Recombinant Full Length Human DYSF Protein, C-Flag-tagged | Mammalian cells | Human | Flag | Full L. |
|
| DYSF-684H | Recombinant Human DYSF Protein, His-tagged | E.coli | Human | His | Met1~Tyr479 |
|
| DYSF-796H | Recombinant Human DYSF Protein, His (Fc)-Avi-tagged | HEK293 | Human | His&Fc&Avi |
|
|
| DYSF-796H-B | Recombinant Human DYSF Protein Pre-coupled Magnetic Beads | HEK293 | Human |
|
Background
What is DYSF protein?
DYSF gene (dysferlin) is a protein coding gene which situated on the short arm of chromosome 2 at locus 2p13. The protein encoded by this gene belongs to the ferlin family and is a skeletal muscle protein found associated with the sarcolemma. It is involved in muscle contraction and contains C2 domains that play a role in calcium-mediated membrane fusion events, suggesting that it may be involved in membrane regeneration and repair. In addition, the protein encoded by this gene binds caveolin-3, a skeletal muscle membrane protein which is important in the formation of caveolae. Specific mutations in this gene have been shown to cause autosomal recessive limb girdle muscular dystrophy type 2B (LGMD2B) as well as Miyoshi myopathy. The DYSF protein is consisted of 2080 amino acids and DYSF molecular weight is approximately 237.3 kDa.
What is the function of DYSF protein?
Its primary function is thought to be aiding in the repair of the sarcolemma when it becomes damaged due to muscle strain. Dysferlin may also be involved in the formation of new muscle fibers (regeneration) and in inflammation, although these functions are less well understood. The protein is a part of the ferlin family and contains C2 domains that are implicated in calcium-mediated membrane processes. Loss-of-function or missense variants in the DYSF gene can lead to dysferlinopathies, which are muscular dystrophies characterized by muscle weakness and wasting, particularly affecting the shoulders, hips, thighs, and upper arms.
DYSF Related Signaling Pathway
Studies have shown that miR-92a-1-5p regulates BeWo cell fusion and hCG secretion by regulating the protein kinase A (PKA) signaling pathway and dysferlin expression. DYSF, as a cytoskeletal protein, is closely related to muscle contraction and relaxation in muscle cells. It maintains the integrity of muscle cell membranes and participates in signal transduction processes through interactions with other proteins and molecules. DYSF interacts with a variety of proteins and molecules that work together to regulate the physiological activity of muscle cells, although the specific signaling pathways have not been elucidated in detail.
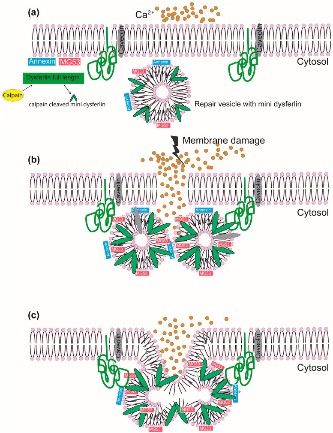
Fig1. The role of dysferlin in membrane repair in a calcium-dependent manner. (Bal Hari Poudel, 2024)
DYSF Related Diseases
The DYSF protein is associated with several hereditary myopathies, primarily including limb band muscular dystrophy 2B (LGMD2B), Miyoshi distal muscular dystrophy, and other myopathies associated with muscle cell membrane repair. These diseases are usually caused by mutations in DYSF genes, resulting in loss or abnormality of DYSF protein function, which in turn causes damage to muscle cell membranes and degeneration or necrosis of muscle cells. The loss or dysfunction of the DYSF protein affects the normal physiological function of muscle cells, leading to symptoms such as muscle weakness and muscle atrophy.
Bioapplications of DYSF
The application of DYSF protein is mainly concentrated in the field of medical research and clinical diagnosis. In terms of research, DYSF protein is a key factor in a variety of hereditary myopathies, and the study of its function and mechanism of action contributes to a deep understanding of the pathological process of these diseases. In addition, the expression and activity of DYSF protein can be used as an important index to study the repair and regeneration process of muscle cell membrane. Clinically, detection of DYSF proteins, such as by immunohistochemistry or western blotting techniques, can help identify and diagnose myopathies associated with DYSF gene mutations, such as limb band muscular dystrophy 2B and Miyoshi distal muscular dystrophy. These tests are important for early diagnosis of the disease, genetic counseling and the development of treatment strategies.
Case Study
Case Study 1: Huahua Zhong, 2021
Dysferlinopathy is one of the most common subgroup of autosomal recessive limb-girdle muscular dystrophies that is caused by mutations in DYSF gene. However, there is currently no worldwide comprehensive genetic analysis of DYSF variants. Through a national multicenter collaborative effort in China, researchers identified 222 DYSF variants with 40 novel variants from 245 patients. They then integrated DYSF variants from disease-related genetic databases including LOVD (n = 1020) and Clinvar (n = 1179), to depict the global landscape of disease-related DYSF variants. Normal-population-derived DSYF variants from gnomAD (n = 4318) and ChinaMAP (n = 13,330) were also analyzed in comparison. In Chinese patients, gender instead of genotype showed influence on the onset age of dysferlinopathy, with males showing an earlier age of onset. However, three specific domains (C2F-C2G-TM, DysF, and C2B-Ferl-C2C) contained variants at higher frequencies than reported in both the databases and Chinese patients.
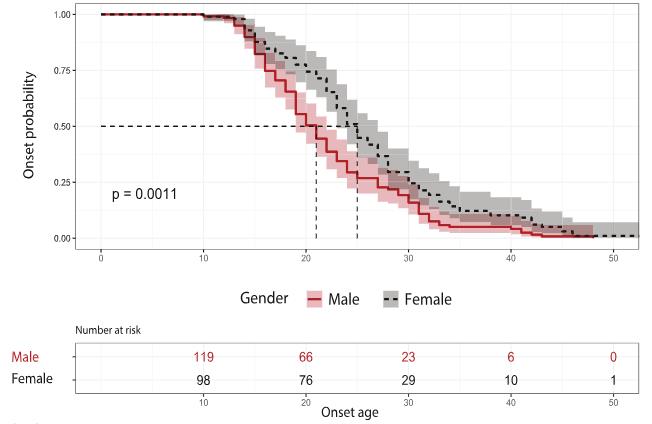
Fig1. The impact of gender on dysferlinopathy onset.
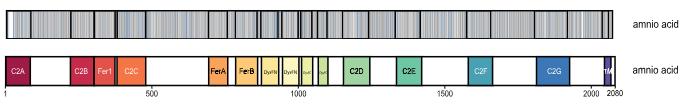
Fig2. The distribution of patient-derived (blue) and normal control (grey) variants on Dysferlin.
Case Study 2: Hiroya Ono, 2020
Mutations in dysferlin are responsible for a group of progressive, recessively inherited muscular dystrophies known as dysferlinopathies. Using recombinant proteins and affinity purification methods combined with liquid chromatography-tandem mass spectrometry (LC-MS/MS), researchers found that AMP-activated protein kinase (AMPK)γ1 was bound to a region of dysferlin located between the third and fourth C2 domains. Using ex vivo laser injury experiments, they demonstrated that the AMPK complex was vital for the sarcolemmal damage repair of skeletal muscle fibers. Injury-induced AMPK complex accumulation was dependent on the presence of Ca2+, and the rate of accumulation was regulated by dysferlin. Furthermore, it was found that the phosphorylation of AMPKα was essential for plasma membrane repair, and treatment with an AMPK activator rescued the membrane-repair impairment observed in immortalized human myotubes with reduced expression of dysferlin and dysferlin-null mouse fibers. Finally, it was determined that treatment with the AMPK activator metformin improved the muscle phenotype in zebrafish and mouse models of dysferlin deficiency.
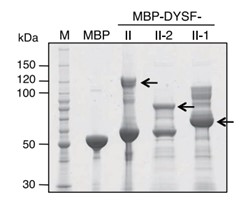
Fig3. Coomassie blue-stained blot of generation of MBP fusion proteins for the II region of dysferlin.

Fig4. Line 107 with dysferlin mutations and the healthy control line KM 155.
Quality Guarantee
High Purity
.jpg)
Fig1. SDS-PAGE (DYSF-4000H)
.
.jpg)
Fig2. SDS-PAGE (DYSF-684H)
Involved Pathway
DYSF involved in several pathways and played different roles in them. We selected most pathways DYSF participated on our site, such as , which may be useful for your reference. Also, other proteins which involved in the same pathway with DYSF were listed below. Creative BioMart supplied nearly all the proteins listed, you can search them on our site.
| Pathway Name | Pathway Related Protein |
|---|
Protein Function
DYSF has several biochemical functions, for example, . Some of the functions are cooperated with other proteins, some of the functions could acted by DYSF itself. We selected most functions DYSF had, and list some proteins which have the same functions with DYSF. You can find most of the proteins on our site.
| Function | Related Protein |
|---|
Interacting Protein
DYSF has direct interactions with proteins and molecules. Those interactions were detected by several methods such as yeast two hybrid, co-IP, pull-down and so on. We selected proteins and molecules interacted with DYSF here. Most of them are supplied by our site. Hope this information will be useful for your research of DYSF.
Resources
Related Services
Related Products
References
- ten Dam, L; van der Kooi, AJ; et al. Comparing clinical data and muscle imaging of DYSF and ANO5 related muscular dystrophies. NEUROMUSCULAR DISORDERS 24:1097-1102(2014).
- Kergourlay, V; Rai, G; et al. Identification of Splicing Defects Caused by Mutations in the Dysferlin Gene. HUMAN MUTATION 35:1532-1541(2014).


