CDA
-
Official Full Name
cytidine deaminase -
Overview
This gene encodes an enzyme involved in pyrimidine salvaging. The encoded protein forms a homotetramer that catalyzes the irreversible hydrolytic deamination of cytidine and deoxycytidine to uridine and deoxyuridine, respectively. It is one of several deaminases responsible for maintaining the cellular pyrimidine pool. Mutations in this gene are associated with decreased sensitivity to the cytosine nucleoside analogue cytosine arabinoside used in the treatment of certain childhood leukemias. -
Synonyms
CDA;cytidine deaminase;CDD;Cytidine aminohydrolase;EC 3.5.4.5;small cytidine deaminase;cytosine nucleoside deaminase
Recombinant Proteins
- Human
- Zebrafish
- Chicken
- E.coli
- Wheat Germ
- Mammalian Cell
- HEK293T
- In Vitro Cell Free System
- E. coli
- His
- GST
- His&T7
- Myc&DDK
- His&Myc
| Cat.# | Product name | Source (Host) | Species | Tag | Protein Length | Price |
|---|---|---|---|---|---|---|
| CDA-796H |
Recombinant Human Cytidine Deaminase, His-tagged

|
E.coli | Human | His | ||
| CDA-3132H | Recombinant Human CDA protein, His-tagged | E.coli | Human | His | 42-146 aa | |
| CDA-0894H | Recombinant Human CDA Protein, GST-Tagged | Wheat Germ | Human | GST |
|
|
| CDA-11703Z | Recombinant Zebrafish CDA | Mammalian Cell | Zebrafish | His |
|
|
| CDA-206H | Recombinant Human CDA protein, T7/His-tagged | E.coli | Human | His&T7 |
|
|
| CDA-26955TH | Recombinant Human CDA, His-tagged | E.coli | Human | His | 146 amino acids |
|
| CDA-317HCL |
Recombinant Human CDA Over-ex |
HEK293T | Human | Myc&DDK |
|
|
| CDA-3925C | Recombinant Chicken CDA | Mammalian Cell | Chicken | His |
|
|
| CDA-1079H | Recombinant Human CDA Protein (Ala2-Gln146), His tagged | E.coli | Human | His | Ala2-Gln146 |
|
| CDA-3108HF | Recombinant Full Length Human CDA Protein, GST-tagged | In Vitro Cell Free System | Human | GST | Full L. 146 amino acids |
|
| CDA-5564H | Recombinant Human CDA protein, His-tagged | E. coli | Human | His | 161aa(including fusion tag) |
|
| CDA-598H | Recombinant Human CDA Protein, His-tagged | E.coli | Human | His | Ala2~Gln146 |
|
| CDA-9381H | Recombinant Human CDA protein, His&Myc-tagged | E.coli | Human | His&Myc | 1-146aa |
|
Background
What is CDA protein?
CDA (cytidine deaminase) gene is a protein coding gene which situated on the short arm of chromosome 1 at locus 1p36. This gene encodes an enzyme involved in pyrimidine salvaging. The encoded protein forms a homotetramer that catalyzes the irreversible hydrolytic deamination of cytidine and deoxycytidine to uridine and deoxyuridine, respectively. It is one of several deaminases responsible for maintaining the cellular pyrimidine pool. CDA protein is consisted of 146 amino acids and its molecular mass is approximately 16.2 kDa.
What is the function of CDA protein?
CDA proteins catalyze the conversion of cytosine (cytidine) and deoxycytidine (deoxycytidine) to uridine (uridine) and deoxyuridine (deoxyuridine), thus participating in maintaining the balance of pyrimidine nucleotides in cells. CDA plays a role in the pyrimidine remediation pathway, helping cells recycle and reuse pyrimidine bases, which is essential for the synthesis of nucleic acids and the normal function of cells. In some cases, CDA proteins may also be involved in the regulation of cell survival, such as affecting the survival of CDA-deficient cells in the presence of Tau co-depletion.
CDA Related Signaling Pathway
CDA proteins catalyze the conversion of cytosine and deoxycytosine to uracil and deoxyuracil, and are involved in maintaining the balance of pyrimidine nucleotides in cells. The activity of CDA proteins may influence the apoptosis process because of its role in regulating nucleotide levels, changes in which can trigger cell death signals. CDA proteins may be involved in the metabolism of drugs, as their role in pyrimidine metabolism may affect the activation or deactivation of drugs. CDA proteins may be involved in cell cycle regulation and DNA repair processes, as their role in maintaining nucleotide pools is critical to these processes.

Fig1. A schematic diagram of the effects and prognostic values of circadian genes MBOAT2/CDA/LPCAT2/ B4GALT5 in pancreatic cancer. (Qingqing Wang, 2023)
CDA Related Diseases
In some cases, abnormal expression of CDA may be associated with the development of anemia, especially diseases related to red blood cell production. CDA is associated with certain types of neutropenia, a condition in which white blood cells are reduced and may be associated with periodic neutropenia and skin heterosis. Certain studies have shown that CDA-associated enzymes, such as members of the APOBEC family, help inhibit viral replication by promoting the deamination of cytosine to uracil and may play a role in cancer.
Bioapplications of CDA
The main application of CDA in drug development is as a target for anticancer drugs. CDA can catalyze the conversion of Cytidine to Uracil, a process that plays a key role in DNA replication and repair. By inhibiting the activity of CDA, DNA repair of cancer cells can be prevented, thus inhibiting their growth and spread. Therefore, CDA inhibitors are considered as a potential anticancer drug. Cdas are also used in gene-editing technologies such as CRISPR-Cas9. In the CRISPR-Cas9 system, CDA can catalyze the conversion of Uracil in the guide RNA (gRNA) to Cytidine, thereby repairing uracil mutations in gRNA and improving the accuracy and efficiency of the CRISPR-Cas9 system.
Case Study
Case Study 1: Audrey Lumeau, 2024
Cytidine deaminase (CDA) functions in the pyrimidine salvage pathway for DNA and RNA syntheses and has been shown to protect cancer cells from deoxycytidine-based chemotherapies. In this study, the researchers observed that CDA was overexpressed in pancreatic adenocarcinoma from patients at baseline and was essential for experimental tumor growth. Mechanistic investigations revealed that CDA localized to replication forks where it increased replication speed, improved replication fork restart efficiency, reduced endogenous replication stress, minimized DNA breaks, and regulated genetic stability during DNA replication. In cellular pancreatic cancer models, high CDA expression correlated with resistance to DNA-damaging agents. Silencing CDA in patient-derived primary cultures in vitro and in orthotopic xenografts in vivo increased replication stress and sensitized pancreatic adenocarcinoma cells to oxaliplatin. This study sheds light on the role of CDA in pancreatic adenocarcinoma, offering insights into how this tumor type modulates replication stress.

Fig1. Western blot analysis of FLAG-CDA in cytosolic and nuclear fractions of MIA PaCa-2 cells.
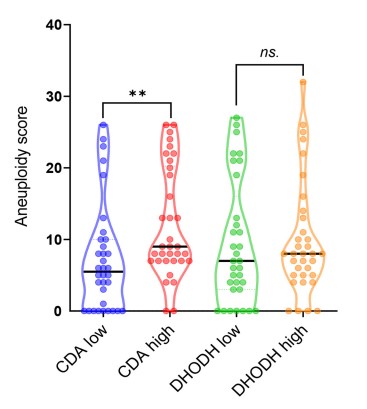
Fig2. Quantification of aneuploidy score in TCGA PDAC samples with high and low level of CDA.
Case Study 2: Appolinaire A Olou, 2020
The Mucin 1 (MUC1) protein is overexpressed in various cancers and mediates chemotherapy resistance. However, the mechanism is not fully understood. Given that most chemotherapeutic drugs disrupt ER homeostasis as part of their toxicity, and MUC1 expression is regulated by proteins involved in ER homeostasis, the researchers investigated the link between MUC1 and ER homeostasis. MUC1 knockdown in pancreatic cancer cells enhanced unfolded protein response (UPR) signaling and cell death upon ER stress induction. Transcriptomic analysis revealed alterations in the pyrimidine metabolic pathway and cytidine deaminase (CDA). ChIP and CDA activity assays showed that MUC1 occupied CDA gene promoter upon ER stress induction correlating with increased CDA expression and activity in MUC1-expressing cells as compared with MUC1 knockdown cells. Inhibition of either the CDA or pyrimidine metabolic pathway diminished survival in MUC1-expressing cancer cells upon ER stress induction. Metabolomic analysis demonstrated that MUC1-mediated CDA activity corresponded to deoxycytidine to deoxyuridine metabolic reprogramming upon ER stress induction.
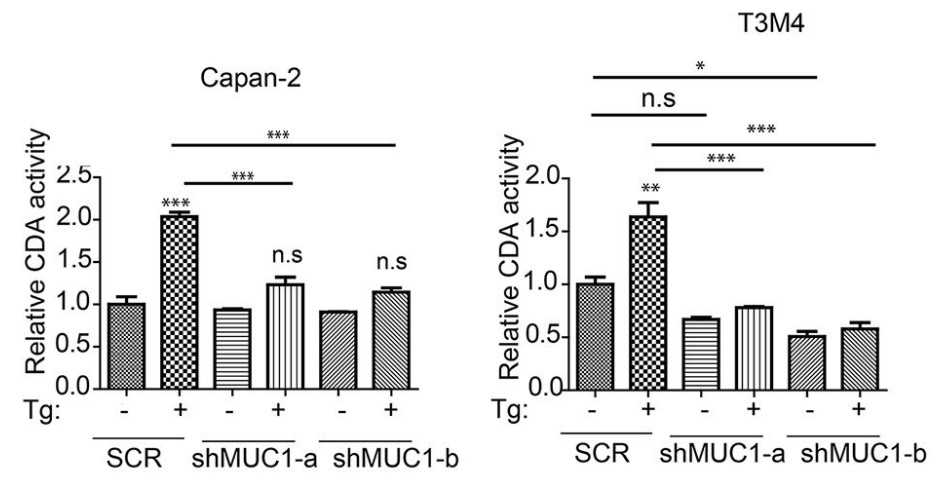
Fig3. CDA activity in SCR and MUC1 knockdown cells relative to SCR.
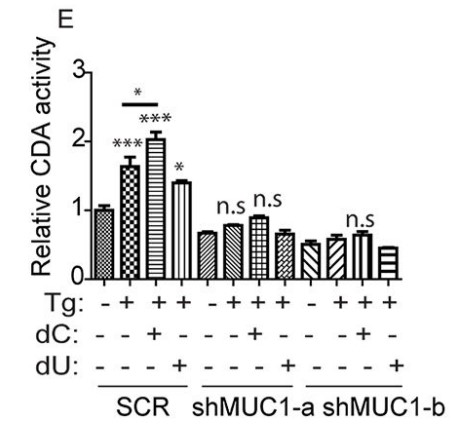
Fig4. CDA activity, relative to SCR, is represented by the bar charts.
Quality Guarantee
High Purity
.jpg)
Fig1. SDS-PAGE (CDA-0894H)
.
.jpg)
Fig2. SDS-PAGE (CDA-598H)
Involved Pathway
CDA involved in several pathways and played different roles in them. We selected most pathways CDA participated on our site, such as Pyrimidine metabolism,Drug metabolism - other enzymes,Metabolic pathways, which may be useful for your reference. Also, other proteins which involved in the same pathway with CDA were listed below. Creative BioMart supplied nearly all the proteins listed, you can search them on our site.
| Pathway Name | Pathway Related Protein |
|---|---|
| Drug metabolism - other enzymes | UGT1A2,DPYD,GUSB,Ces2c,UCK2A,IMPDH1B,CES1,UGT2A3,IMPDH1,UGT2B15 |
| Pyrimidine metabolism | UCK1,ITPA,POLR2J2,NT5C1B-RDH14,POLR2J3,AK9,NME2A,UCK2,ENTPD4,UCKL1 |
| Metabolic pathways | UGDH,PPT1,PNAT3,LIPCA,COX6C,AGPAT9,PRPS1B,AKR1A1A,FECH,GALNTL4 |
Protein Function
CDA has several biochemical functions, for example, cytidine deaminase activity,nucleoside binding,protein binding. Some of the functions are cooperated with other proteins, some of the functions could acted by CDA itself. We selected most functions CDA had, and list some proteins which have the same functions with CDA. You can find most of the proteins on our site.
| Function | Related Protein |
|---|---|
| zinc ion binding | USP51,PPARD,DNPEP,CCS,RORAB,SETDB1,ZNRF1,ADAM22,KDM5BB,PTER |
| protein binding | TP53INP1,EZH2,TGFBI,MRPS9,YY1AP1,MTCH1,USF2,HIST1H1A,BCDIN3D,SMAD4 |
| cytidine deaminase activity | WBP2NL,APOBEC3H,APOBEC3F,APOBEC3G,APOBEC2,AICDA,APOBEC1 |
| nucleoside binding | ACTN4,AASS,PNP,POLA1 |
| protein homodimerization activity | TRIM5,SP1,IL17F,IZUMO1,AMBP,GSS,PDCD6IP,ALS2,NCR3,PYCARD |
Interacting Protein
CDA has direct interactions with proteins and molecules. Those interactions were detected by several methods such as yeast two hybrid, co-IP, pull-down and so on. We selected proteins and molecules interacted with CDA here. Most of them are supplied by our site. Hope this information will be useful for your research of CDA.
SDCBP;ZBTB24;PSMA1;PTGER3;LNX1;PLEKHB2;WDYHV1
Resources
Related Services
Related Products
References
- Jansen, MA; Tromp, IIM; et al. Infant feeding and anti-tissue transglutaminase antibody concentrations in the Generation R Study. AMERICAN JOURNAL OF CLINICAL NUTRITION 100:1095-1101(2014).
- Topp, ZZ; Saven, A; et al. Hairy cell leukemia: a 'hair-raising' update. EXPERT REVIEW OF HEMATOLOGY 7:659-669(2014).


