Endotoxin Removal Service
At Creative BioMart, we make it our mission to eliminate those endotoxins from your samples. It doesn't matter if you're dealing with proteins, antibodies, vaccines, nucleic acids, buffers, or cell extracts—we've got it under control. We customize our service to ensure high recovery rates, tweaking the process to match the unique characteristics and application demands of each sample. With years of experience, we've perfected our specialized protocols to meet tight endotoxin standards, providing reliable support for all your research and application projects.
Background
Importance of Endotoxin Removal
Endotoxins, known as lipopolysaccharides, are key components in the cell walls of Gram-negative bacteria. They often find their way into numerous biological products and pose significant health risks, especially in the realms of biopharmaceuticals and clinical research. Endotoxins can trigger fierce immune responses, causing fever, inflammation, and sometimes severe conditions like sepsis. That's why monitoring and getting rid of these endotoxins is crucial in the production and research of biopharmaceuticals to ensure the safety and efficacy of the products.
Getting rid of endotoxins is essential when you're preparing biological products intended for clinical use. High levels of endotoxins can raise serious safety issues, which could hold back product marketability and clinical use. Moreover, eliminating endotoxins precisely can increase the reliability of experimental data by minimizing interference from these contaminants. Thanks to Creative BioMart's removal service, researchers and producers can be confident that their products meet the strict international safety standards, ultimately boosting the quality and safety of their bioproducts.

Applications of this Service
- Biopharmaceutical Development
When it comes to biopharmaceuticals, kicking out endotoxins is super important. In drug development, particularly with stuff like proteins, antibodies, and vaccines, you need to hit those tough safety and purity marks. Our service for removing endotoxins makes sure your products stay on track during the development phase and afterwards, boosting their overall safety and effectiveness.
- Clinical Research and Diagnostic Tool Preparation
Removing endotoxins is also vital in preparing tools for clinical research and diagnostics. Ensuring that samples are free from endotoxins reduces immune reactions and enhances the accuracy of diagnostics and research results. This is essential for producing high-quality diagnostic reagents and devices, ensuring they are safe for clinical use.
- Academic Research
In academic settings, especially in molecular biology and biochemistry, endotoxins can mess with the reliability of your experiments. By clearing them out, you can enhance the accuracy of your experiments and ensure your data is spot-on. This helps researchers draw more reliable conclusions and pushes scientific knowledge forward.
Service Details
Service Process
What We Offer?
- Innovative Techniques We Offer
We tackle endotoxin removal with top-notch methods like affinity chromatography and custom filtration systems. Our services are all about efficiently clearing lipopolysaccharides from your samples. We choose the best techniques to fit what each sample specifically requires, making sure everything is spot on for your needs.
- Comprehensive Sample Handling
Our services cover a wide range of sample types, including proteins, antibodies, vaccines, nucleic acids, and cell extracts. We have the expertise and equipment to ensure that all these samples are free from endotoxins. Our team develops customized strategies to address the unique properties of each sample, delivering optimal results for your specific requirements.
- Precision and Compliance Assurance
We are committed to offering precise endotoxin removal services that achieve over 99.99% reduction levels, with detection capabilities as sensitive as 0.005 EU/ml. We ensure endotoxin levels are brought down to below 0.1 EU/ml, meeting even the most rigorous international safety standards. This ensures that your samples are thoroughly prepared and safe for all downstream applications or analyses.
Online InquiryWhy Choose Us?
Case Study
Case 1: TEV Protease: Endotoxin-Free Tag Removal
In recent findings, researchers explored the expression of Tobacco Etch virus (TEV) protease in Bacillus subtilis, a GRAS (Generally Recognized As Safe) host strain, to effectively remove fusion tags without endotoxin contamination. By creating a fusion protein that includes a lysyl-tRNA synthetase domain and TEV protease, scientists managed to achieve high-level expression in B. subtilis. Using IPTG induction, they successfully overexpressed His-TEV protease, which showed excellent activity in cleaving fusion proteins. The resulting products were purified through a Ni-NTA column, ensuring an endotoxin-free outcome. This method not only promises clean tag removal but also stands as a viable option for producing therapeutic proteins and advancing biomedical applications.
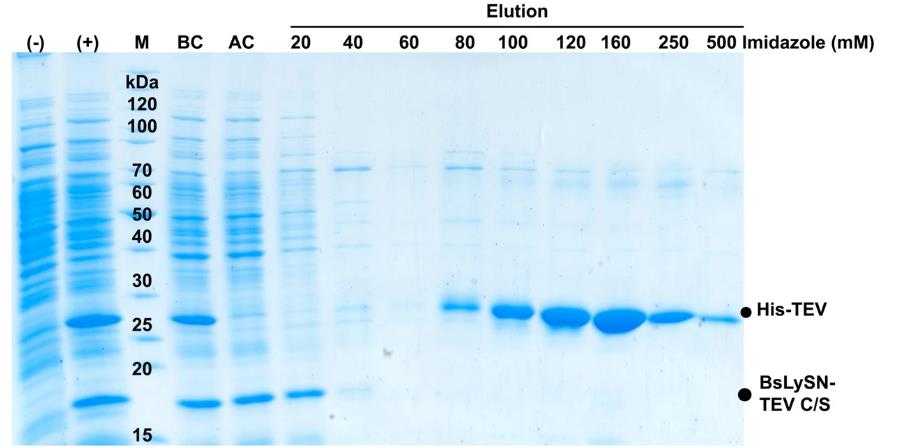 Fig. 1. Purification of His-TEV protease using Ni–NTA columns. (Le VD, et al. Curr Microbiol. 2024)
Fig. 1. Purification of His-TEV protease using Ni–NTA columns. (Le VD, et al. Curr Microbiol. 2024)Case 2: Precise Endotoxin Removal Using Anti-Lipid A Microparticles for Enhanced Drug Formulation
Endotoxins, like lipopolysaccharides (LPS) from Gram-negative bacteria, can trigger severe immune responses. Injectable drugs must adhere to strict endotoxin limits, requiring LPS removal. Typical methods non-specifically bind LPS to charged or hydrophobic surfaces, sometimes removing vital proteins, too. Researchers developed microparticles with anti-Lipid A antibodies for precise endotoxin removal, analyzed using flow cytometry and microscopy. These particles removed over 6,000 endotoxin units per mg from water, verified by the Limulus Amebocyte Lysate test and nanoparticle tracking analysis. Compared to general removal systems, these specific particles better preserved proteins across formulations-bovine serum albumin, insulin, and birch pollen extracts-suggesting improved product yields.
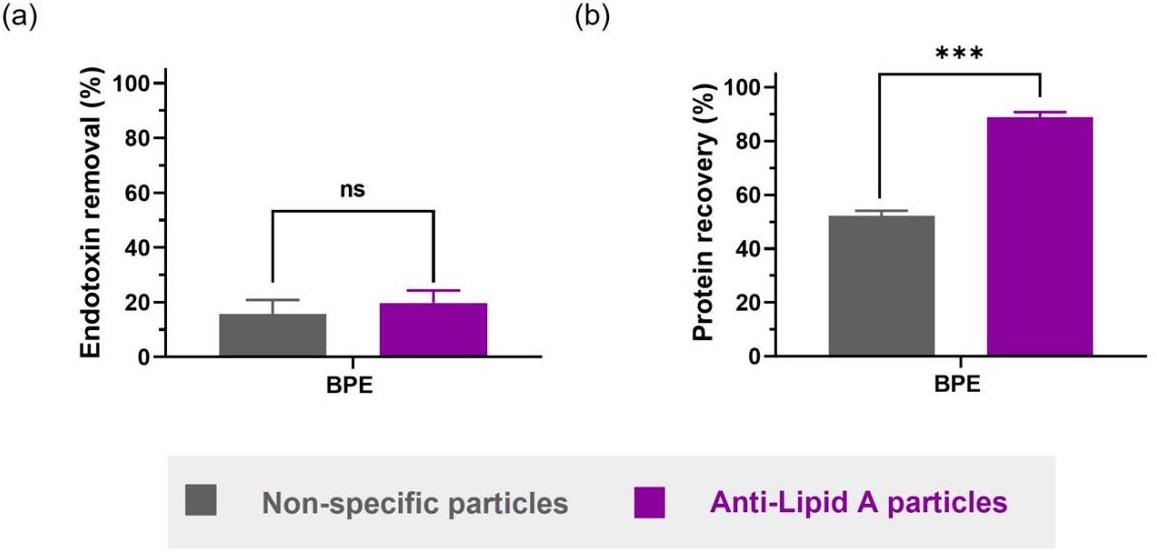 Fig. 2. Comparison between anti-Lipid A particles and commercial non-specific particles in BPE in DPBS regarding (a) endotoxin removal and (b) protein recovery. (Casonato, et al. 2023)
Fig. 2. Comparison between anti-Lipid A particles and commercial non-specific particles in BPE in DPBS regarding (a) endotoxin removal and (b) protein recovery. (Casonato, et al. 2023)Case 3: Efficient Endotoxin Removal from Protein Nanocages for Clinical Applications
Protein nanocages are gaining attention as promising delivery systems due to their uniform structure, stability in biological fluids, and ability to be customized and loaded with various drugs. However, producing these nanocages in E. coli often results in high endotoxin contamination, causing immune reactions. Removing endotoxins while keeping protein stability is crucial, especially since injectable formulations need low endotoxin levels for clinical testing (<5 EU/kg). Typical methods like affinity chromatography or detergents don't work well for protein nanocages. Here researchers have developed a protocol that combines affinity purification using Endotrap HD resin with Triton X-114 treatment to clean human H-ferritin nanocages. This method achieves high purity and maintains protein recovery without affecting how nanocages target cells. Tests confirm that purified H-ferritin still specifically binds to cancer cells, paving the way for clinical trials.
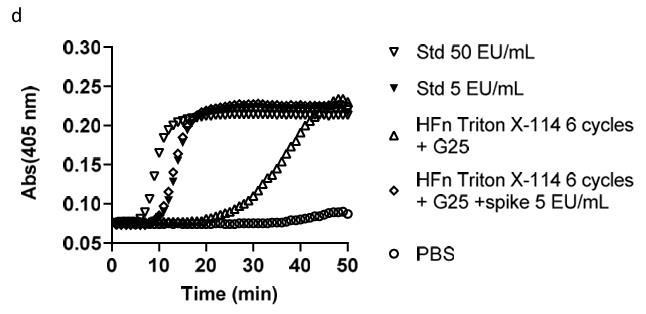 Fig. 3. Representative turbidimetric plots of HFn purified with 6 cycles of Triton X-114. (Silva, et al. 2021)
Fig. 3. Representative turbidimetric plots of HFn purified with 6 cycles of Triton X-114. (Silva, et al. 2021)FAQs
-
Q: What makes your company's endotoxin removal technique stand out?
A: Targeted Approach: We use unique microparticles that effectively remove endotoxins while keeping essential proteins intact.
Personalized Solutions: We adjust our methods to fit the specific needs of different proteins, ensuring great results even in complex mixes.
Comprehensive Testing: We employ tools like LAL tests and nanoparticle tracking to ensure endotoxins are gone, offering detailed reports for confirmation. -
Q: How do you ensure endotoxins are effectively removed?
A: We use trusted methods such as LAL testing and nanoparticle analysis to verify endotoxin removal, and we provide detailed reports to back it up.
-
Q: How long does the endotoxin removal process take?
A: The timing depends on the sample's characteristics and size, but we typically complete it within a few days after receiving the sample.
-
Q: Do you provide small-scale endotoxin removal trials?
A: Yes, we do small trial runs so clients can see the results firsthand and determine if our approach suits their needs.
-
Q: Do you offer additional purification and analysis services after endotoxin removal?
A: Yes, in addition to endotoxin removal, we provide full protein purification, analysis, and quality control services. You can click here to learn more.
References:
- Singh A.; et al. Protein recovery from inclusion bodies of Escherichia coli using mild solubilization process. Microb Cell Fact. 2015;14:41.
- Casonato Melo C.; et al. Recovering What Matters: High Protein Recovery after Endotoxin Removal from LPS-Contaminated Formulations Using Novel Anti-Lipid A Antibody Microparticle Conjugates. Int J Mol Sci. 2023;24(18):13971.
- Silva F.; et al. Combined Method to Remove Endotoxins from Protein Nanocages for Drug Delivery Applications: The Case of Human Ferritin. Pharmaceutics. 2021;13(2):229.
Contact us or send an email at for project quotations and more detailed information.
Quick Links
-

Papers’ PMID to Obtain Coupon
Submit Now -

Refer Friends & New Lab Start-up Promotions
