Uncategorized Monday, 2025/01/20
Pancreatic cancer is the third leading cause of cancer-related death worldwide. Its five-year survival rate is as low as 10%. It is a malignant tumor with an extremely poor prognosis. Pancreatic ductal adenocarcinoma (PDAC) is the most common histological type of pancreatic cancer, accounting for the vast majority of all cases. Research has found that over 90% of PDAC patients have KRAS gene activation mutations (KRAS mutations), which are not only closely related to tumor formation and progression, but have also long been considered an “undruggable” target.
Although there have been some breakthroughs in clinical trials for therapies targeting KRAS mutations in recent years with advances in molecular biology technology, the overall therapeutic effect of PDAC is still not ideal. Most patients are already in the late stage of the disease (such as locally advanced stage or metastatic stage) at the time of diagnosis, and cannot undergo surgical resection. They mainly rely on chemotherapy to maintain survival. However, how to accurately predict the prognosis of patients and optimize treatment plans remains one of the key challenges in PDAC research.
In this context, Nature Medicine reported a study titled “Clinicogenomic landscape of pancreatic adenocarcinoma identifies KRAS mutant dosage as prognostic of overall survival”. Researchers conducted a comprehensive analysis of clinical and genomic data from 2336 PDAC patients, revealing an important association between KRAS mutant allele dose and disease progression as well as patient survival. Research has found that the dose gain of KRAS mutant alleles is not only a marker of disease progression, but also significantly affects the overall survival of patients. In addition, for patients with KRAS wild type, studies have also identified potential roles of other carcinogenic pathways, providing new ideas for clinical treatment.
This study not only provides a new perspective for the molecular typing and prognostic evaluation of PDAC, but also lays the foundation for the development of more precise targeted therapies. In the future, with the further development of KRAS-targeted therapy, these findings may change the diagnosis and treatment pattern of pancreatic cancer and bring new hope to patients.
The silent killer of pancreatic cancer: status quo and challenges
Pancreatic cancer is known as the “king of cancer”. Its concealment and high mortality make it one of the great challenges facing modern medicine. According to global statistics, pancreatic cancer has climbed to the third leading cause of cancer-related deaths, with a five-year survival rate of only about 10%. Although the incidence rate of this malignant tumor is relatively low, its prognosis is extremely poor, which brings a heavy psychological and economic burden to patients and their families.
Main characteristics of PDAC
PDAC is the most common type of pancreatic cancer, accounting for the vast majority of all cases. Research has found that the molecular characteristics of PDAC are highly complex, with over 90% of cases having mutations in the KRAS gene. The incidence of this mutation is much higher than other tumor types, indicating its crucial role in disease occurrence and progression. In addition, mutations in genes such as TP53, CDKN2A, and SMAD4 are also common in PDAC patients, providing a deeper molecular basis for the malignant characteristics of tumors.
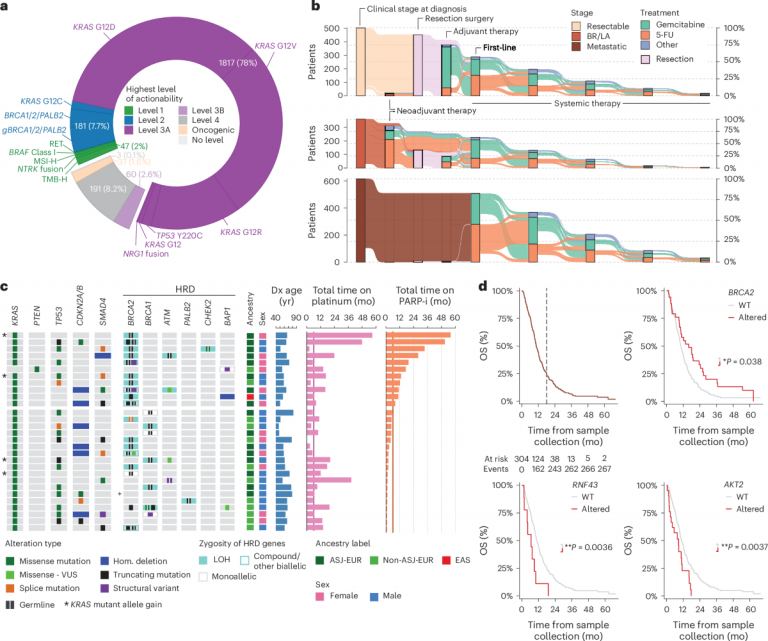
In clinical practice, the characteristics of PDAC include unclear early symptoms, diagnosis usually in the late stage, and strong resistance to treatment. Research shows that among 2336 patients, 44% of cases had distant metastasis at the time of diagnosis, and only 31% of patients had tumors that could be surgically removed. This late diagnosis rate severely limits treatment options and directly affects patient survival rates.
Difficulties in diagnosis and treatment
The high mortality rate of PDAC is not only related to its invasive biological characteristics, but also closely related to the limitations of current diagnosis and treatment. Since the pancreas is located deep in the abdominal cavity, tumors are often difficult to detect in the early stages. When patients show obvious symptoms, they often have already entered the middle and late stages of the disease. In addition, pancreatic tissue is closely related to important blood vessels and organs, making surgery difficult. Small residual tumors at the cutting edge may also lead to postoperative recurrence. In clinical data, only about 41% of patients underwent resection surgery, and their survival was significantly better than those who did not undergo surgery.
In terms of treatment, chemotherapy remains the primary choice for PDAC patients. However, this malignant tumor has limited sensitivity to chemotherapy drugs, and there are significant differences in efficacy among individual patients. Research suggests that some molecular subtypes (such as BRCA1/2 mutations) may be more sensitive to specific treatments, but overall the therapeutic effect of PDAC is still unsatisfactory. In recent years, although targeted therapies targeting KRAS gene mutations have shown signs in clinical trials, they have not yet been widely applied in clinical practice.
KRAS gene mutation: a molecular switch in pancreatic cancer
KRAS gene (Kirsten rat sarcoma viral oncogene homolog), as a proto-oncogene, plays a crucial role in the occurrence and progression of pancreatic cancer. Its mutation not only constitutes the molecular basis of PDAC, but is also considered a key target for exploring the disease’s mechanisms and treatment strategies.
Our Related Proteins
Basic Functions of KRAS Gene and Its Role in Pancreatic Cancer
The KRAS gene encodes a small molecule GTPase that plays a central role in cellular signaling. Under normal circumstances, KRAS protein is activated upon binding to GTP, driving downstream signaling pathways such as the RAS-RAF-MEK-ERK pathway to regulate cell proliferation, differentiation, and survival. When GTP is hydrolyzed into GDP, KRAS protein becomes inactive and signal transduction stops. However, mutations in the KRAS gene, especially point mutations in codon 12, can cause the KRAS protein to remain activated, leading to abnormal cell proliferation and the formation of tumors.
Research shows that more than 90% of PDAC patients carry activated mutations of the KRAS gene, which makes it the main driving gene for pancreatic cancer. These mutations not only initiate tumor formation, but also endow it with stronger invasion ability and drug resistance, which is one of the important reasons for the poor prognosis of PDAC.
The distribution of KRAS mutations and their relationship with tumor progression
The frequency of KRAS mutations is extremely high in PDAC, manifested by differences in distribution and function among different subtypes. The data shows that in PDAC patients, the most common KRAS mutation sites are concentrated at codon 12, including G12D (41%), G12V (32%), and G12R (16%). These mutations have different molecular characteristics: for example, G12D mutations can easily activate the transcription program of the RAS pathway. Although these site mutations are located on the same gene, their biological effects and clinical prognosis may differ.
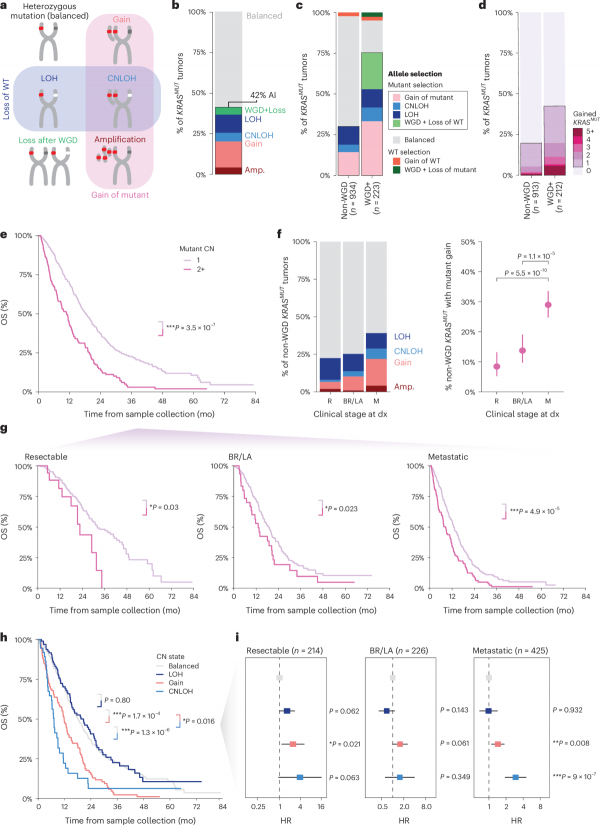
In addition, the dose-response of KRAS mutations has a significant impact on tumor progression and patient survival. Research has found that approximately 42% of KRAS mutant tumors exhibit allelic imbalance, meaning that the number of mutant alleles is higher than that of wild-type alleles. This dose gain is closely related to late-stage disease manifestations, especially in patients who experience whole genome doubling (WGD), where the incidence of this imbalance is as high as 75%. Further analysis shows that patients with KRAS mutation dose gain have significantly shortened overall survival, regardless of disease stage, and this trend is consistent.
Characteristics of KRAS wild-type patients
Although KRAS mutations are highly common in PDAC, about 5% of patients still do not have KRAS mutations detected, becoming so-called KRAS wild type (KRASWT) patients. This population exhibits significant differences in molecular and clinical characteristics.
In KRASWT patients’ tumors, oncogenic mutations in other MAPK pathway genes are common, such as BRAF, NTRK1, and NTRK3. The changes in these genes provide another mechanism for the activation of the MAPK signaling pathway, allowing KRASWT patients to form unique subtypes at the molecular level. In addition, KRASWT patients were diagnosed at an earlier age (median of 58 years, compared to 67 years for KRAS mutation patients) and showed better survival outcomes. This discovery emphasizes the close link between KRAS mutations and disease progression, while also suggesting that KRASWT patients may have different oncogenic pathways and therapeutic targets.
Genomics-based classification of pancreatic cancer
With the advancement of genomics technology, researchers are gradually revealing the molecular basis of PDAC. Through in-depth research on KRAS genes and related signaling pathways, PDAC has been classified into three major genomic subtypes: KRAS mutant (KRASMUT), other MAPK mutant (other MAPKMUT), and MAPK wild type (MAPKWT). These subtypes not only reflect the carcinogenic mechanism of tumors, but also reveal significant differences in patient survival prognosis.
Definition and distribution of the three major subtypes
At the genomic level, PDAC typing is mainly based on the mutation status of the MAPK signaling pathway:
KRAS mutant type (KRASMUT): accounting for the vast majority (95%) of PDAC cases, characterized by point mutations at codon 12 of the KRAS gene, including G12D, G12V, and G12R mutations. These mutations drive tumor formation and progression by continuously activating the RAS-ERK signaling pathway.
Other MAPKMUT gene mutations: account for about 3%, while the KRAS gene remains wild-type, but carcinogenic mutations exist in MAPK pathway genes such as BRAF, NTRK1, and NTRK3, etc. These types of tumors activate the same signaling pathway through alternative mechanisms.
MAPK wild-type (MAPKWT): only 2%, without KRAS mutations or significant mutations in other MAPK pathway genes. This subtype may be involved in non-MAPK pathway-driven tumor mechanisms, and specific features are still under further investigation.
Clinical and molecular characteristics of each subtype
There are significant differences in the molecular characteristics and clinical manifestations of the three subtypes:
KRASMUT subtype: distributed in various stages of the disease, but the increase in mutation dose is highly correlated with advanced disease. This subtype is often associated with high mutation rates in genes such as TP53 (78%) and CDKN2A/B (60%), indicating higher invasiveness and poorer prognosis.
Other MAPKMUT subtype: The BRAF mutation in this subtype has molecular characteristics similar to the KRAS mutation, but the enrichment of ARID1A (21%) and other gene mutations is higher in tumor tissue. In addition, the median age of diagnosis for patients (64 years) was lower compared to KRASMUT patients, indicating the possibility of earlier pathogenic factors.
MAPKWT subtype: The diagnosis age of patients further decreases (median 58 years), and the proportion is slightly higher in the East Asian population (4.8%). In this subtype, the frequency of non-MAPK gene mutations such as GNAS and PIK3CA is higher, suggesting that its carcinogenic mechanism may be related to other pathways.
Survival differences among patients of different subtypes
The survival prognosis of PDAC patients is significantly influenced by molecular subtypes. In multivariate Cox regression analysis, KRASMUT patients had the shortest survival time, while other MAPKMUT and MAPKWT patients showed better survival prognosis. In all clinical stages, the survival of MAPKWT patients was significantly higher than that of KRASMUT patients (adjusted hazard ratio HR’adj=0.69, P=0.041). Especially for patients without targeted therapy, the difference in survival between KRASMUT and other MAPKMUT subtypes tends to disappear, while the survival advantage of MAPKWT patients remains significant (HR’adj=0.68, P=0.035).
The typing based on KRAS mutations and their related pathways not only reveals the molecular heterogeneity of PDAC, but also provides important clues for personalized treatment. For example, KRASWT patients may benefit from targeted therapy targeting BRAF or NTRK mutations, while the treatment of KRASMUT patients should focus more on the impact of mutation dosage.
KRAS mutation dose: a new prognostic marker
The invasiveness and treatment challenges of pancreatic ductal adenocarcinoma (PDAC) require researchers to continuously explore new molecular markers to better evaluate disease progression and patient prognosis. In the context of widespread mutations in the KRAS gene, studies have shown that changes in KRAS mutant allele dose are an important prognostic indicator closely related to tumor progression and patient survival.
The dose of KRAS mutation refers to the proportion of KRAS mutant alleles in a tumor. Under normal circumstances, the KRAS gene exists in the form of alleles in cells, one from each parent. In KRAS mutant type (KRASMUT) PDAC, if the number of mutant alleles is significantly higher than that of wild-type (WT) alleles, it is called a mutant allele gain.
This dose gain is not simply a result of genetic mutations, but rather accumulates through various mechanisms such as genome duplication and allele imbalance. The increase in KRAS mutation dose is often accompanied by enhanced tumor invasiveness and increased tolerance to treatment, making it an important driving force for disease progression.
The association between KRAS mutation dose and disease staging and prognosis
The analysis of genomic data from 2336 PDAC patients showed that approximately 42% of KRASMUT tumors exhibited dose gain due to KRAS mutations, and this phenomenon was more common in the late stages of the disease. For example, the incidence of KRAS mutation dose gain is 8% in resectable tumor patients, rising to 14% in locally advanced patients, and as high as 29% in metastatic patients.
Further multivariate analysis showed a significant correlation between KRAS mutation dose and overall survival (OS) of patients. For example, in patients who did not experience whole genome duplication (WGD), the increase in mutation dose increased the patient’s survival risk by 70% (HR’adj=1.7, P=3.5 × 10 ⁻⁷). Specifically, in patients with resectable tumors, the median survival of those with KRAS mutation dose gain was 23 months, while those without gain had a median survival of 32 months. In metastatic patients, this gap is more significant (8.5 months vs. 13 months). These data indicate that changes in KRAS mutation dosage are not only indicative of disease staging, but also a core indicator of poor prognosis.
The molecular mechanism behind the dose variation of the KRAS gene
The variation in KRAS mutation dose is usually due to the selective amplification of mutant alleles by tumor cells. The main mechanisms include:
Genome Doubling (WGD): WGD is a common genomic event in advanced cancer that leads to a doubling of chromosome numbers. In WGD tumors, the incidence of KRAS mutation dose gain skyrocketed from 30% in non-WGD tumors to 75%, leading to a more severe prognosis.
Allelic imbalance: including amplification of KRAS mutant alleles or loss of wild-type alleles. It is worth noting that research has found that the increase in KRAS mutation dose has a synergistic effect with the loss of wild-type alleles, further exacerbating the malignant nature of the disease.
Mutation-specific effects: Different types of KRAS mutations (such as G12D, G12V, etc.) may have different functional effects under dose gain, such as higher signal pathway activation intensity or metastatic potential.
The Hope of Targeting KRAS: A Breakthrough in Future Treatment
The frequent occurrence of KRAS gene mutations in PDAC has made it a coveted target for researchers. However, this field has long been seen as an undruggable challenge. With the emergence of new technologies and ideas, targeted treatment of KRAS is gradually becoming an important breakthrough in overcoming PDAC.
The traditional treatment dilemma of KRAS mutations
The sustained activation caused by KRAS gene mutations results in an abnormally strong downstream RAS-ERK signaling pathway, driving unlimited proliferation of tumor cells and resistance to treatment. However, the unique structure of KRAS protein and the lack of targeted pockets and binding sites have long hindered drug development. In addition, the complex function of KRAS and its cross-interaction with multiple signaling pathways also increase the challenges of targeted therapy.
Chemotherapy is currently the main treatment option for PDAC patients, but its effectiveness is limited. Even with first-line treatments such as FOLFIRINOX or gemcitabine combination therapy, the median survival of patients is still relatively short. The difficulty in developing directly targeted drugs for KRAS has led researchers to attempt interventions in its downstream pathways, such as MEK or ERK inhibitors. However, these attempts have not been widely applied due to lack of efficacy or excessive side effects.
Emerging KRAS targeted therapies and their potential
In recent years, breakthroughs have been made in the field of KRAS-targeted therapy. The emergence of KRAS G12C inhibitors provides a new approach for targeting specific KRAS mutations. These drugs can specifically bind to the active site of KRAS G12C mutant protein, inhibiting its function. At present, significant results have been achieved in clinical trials of KRAS G12C inhibitors in non-small cell lung cancer, and preliminary data from PDAC patients are also encouraging.
In addition, some multifunctional targeted drugs are also under development. For example, specific RAS inhibitors and molecules that induce mutant KRAS degradation (PROTACs) have shown potential. Another promising approach is combination therapy, such as the combination of KRAS inhibitors with immune checkpoint inhibitors or DNA repair targeted drugs, to enhance therapeutic efficacy.
The study also found that KRAS wild-type (KRASWT) PDAC patients may benefit from specific targeted therapies due to the presence of other targetable mutations (such as BRAF, NTRK) in their tumors. This discovery further broadens the scope of treatment adaptation and provides new hope for patients.
The possibility of optimizing treatment based on KRAS dosage information
The discovery of KRAS mutation dosage has brought more possibilities for personalized treatment. Research has shown that the dose gain of KRAS mutations is closely related to the invasiveness and treatment resistance of the disease. Incorporating KRAS dosage information into treatment decisions may help clinicians to more accurately select treatment plans for patients.
For example, for patients with lower mutation doses, standard chemotherapy may be sufficient to control the condition, while patients with high-dose mutations require more aggressive treatment strategies such as KRAS-targeted drugs or combination therapy. Furthermore, dynamic monitoring based on liquid biopsy can provide real-time evaluation of KRAS dose changes and provide a basis for adjusting treatment plans.
With the gradual maturity of KRAS targeted therapy, a comprehensive treatment plan combining genomics and dynamic monitoring is expected to fundamentally change the treatment prospects of PDAC patients. KRAS, once a barrier to treatment, is now becoming the key to conquering this deadly disease. The rapid development of this field not only brings more hope for survival to patients, but also sets a new benchmark for the future of precision medicine.
Related Products and Services
Protein Expression and Purification Services
Reference
Varghese, A. M., Perry, M. A., Chou, J. F., Nandakumar, S., Muldoon, D., Erakky, A., Zucker, A., Fong, C., Mehine, M., Nguyen, B., Basturk, O., Balogun, F., Kelsen, D. P., Brannon, A. R., Mandelker, D., Vakiani, E., Park, W., Yu, K. H., Stadler, Z. K., . . . M., E. (2025). Clinicogenomic landscape of pancreatic adenocarcinoma identifies KRAS mutant dosage as prognostic of overall survival. Nature Medicine, 1-12. https://doi.org/10.1038/s41591-024-03362-3

