Uncategorized Sunday, 2025/04/13
ADC drugs refer to monoclonal antibodies linked to cytotoxic small molecule payloads through covalent linkers to achieve targeted drug delivery. As of now, more than 11 ADC drugs have been approved for the market.
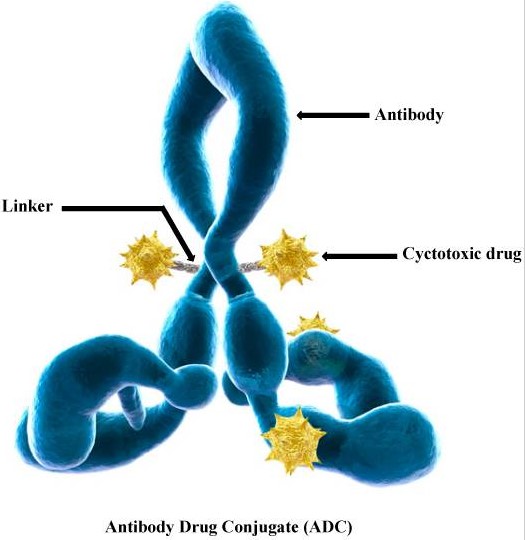
Mechanism of Targeted Therapy in ADC Drugs
Traditional Mechanism
The antibody binds to antigens on the surface of target cells.
It enters the lysosome through endocytosis, where enzymes or acids degrade the ADC, releasing the payload.
The cytotoxic payload kills the tumor cells, achieving high selectivity in anti-tumor effects.
In some cases, the payload has strong permeability, allowing it to diffuse out of the cells and kill bystander tumor cells.
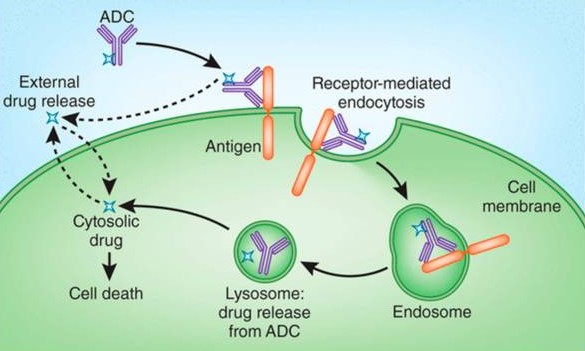
Non-Internalization Mechanism
The linker cleaves in the tumor microenvironment outside the cells, releasing the payload without the need for endocytosis, enabling the selection of non-internalized antigens as targets.
Criteria for Selecting Linkers
Linkers serve as connecting segments between mAbs and payloads, playing a crucial role in the drug’s toxicity and activity. Ideally, linkers should meet the following criteria:
- Have sufficient stability for in vivo transportation and diffusion.
- Require high solubility to facilitate bioconjugation and prevent insoluble aggregates that lead to inactivation.
- Possess adequate cleavage capability to efficiently release payloads at the target site.
Types and Cleavage Mechanisms of Linkers
Currently, common linkers are mainly divided into two categories based on their cleavage mechanisms: chemical cleavage and enzymatic cleavage.
Chemical Cleavage Linkers
Acid-Cleavable Linkers
These linkers need to be stable in the blood transport environment (pH=7.4) and cleave in acidic environments such as the endosome (pH=5.5-6.2) and lysosome (pH=4.5-5.0) of cells.
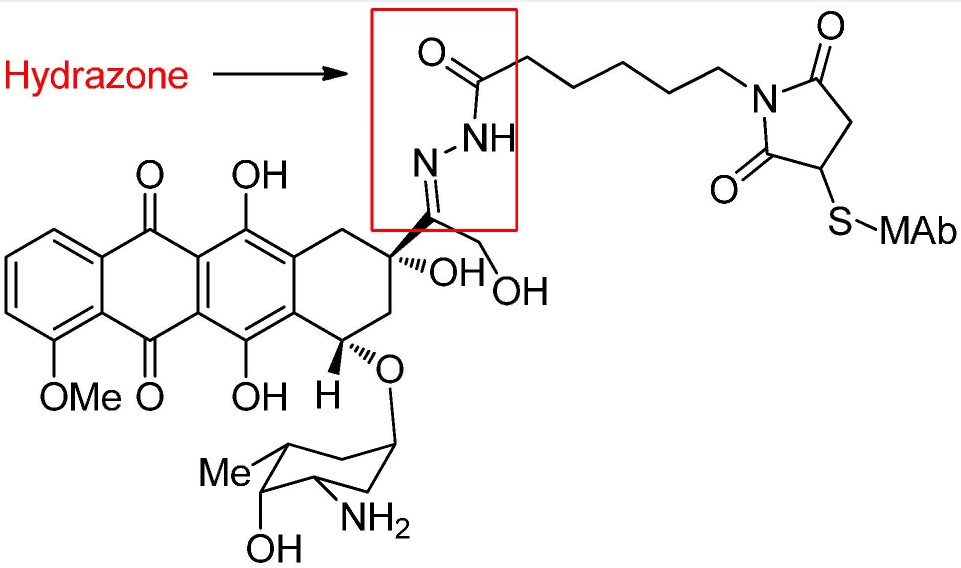
In Mylotarg, a hydrazone linker is acid-hydrolyzed into ketone and hydrazine, effectively removing the linking segment and releasing the drug. Disulfide bonds are also used in this ADC.
Another common acid-labile structure is carbonate-based linkers. Simple carbonates have limited stability in serum and need the addition of a p-aminobenzyl group (PAB) to significantly enhance the half-life. Carbonate structures release SN-38 and carbon dioxide under acidic conditions.
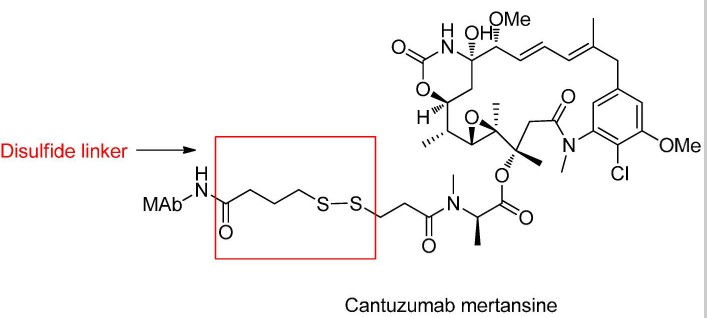
Disulfide Bond Chemical Cleavage
Disulfide compounds are stable under physiological pH conditions but easily undergo nucleophilic attack by thiols, resulting in cleavage of the linkage.
In plasma, the main thiol source is reduced HSA, though the thiol groups in these macromolecules are typically buried, showing poor reactivity.
The cytoplasm contains abundant glutathione (GSH), with higher concentrations in cancer cells, which can efficiently cleave disulfide bonds.
Sterically Hindered Disulfide Bonds
The stability of disulfide bonds is easily influenced by α-substituents, affecting ADC’s physiological activity.
Maytansinoid-ADCs target HER2+ ADC drugs. Researchers explored the activity of disulfide bond cleavage with methyl substituents at the α-position.
It was found that as the substituents increased, disulfide bonds became very inert.
Unsubstituted disulfide bonds cleave easily, whereas disubstituted bonds scarcely release drugs. Only mono-substituted structures exhibited maximum pharmacological activity (R1=H, R2=Me, DM1).
Direct Coupling of Disulfide Bonds to Antibody Load
Disulfide bonds can form directly between cysteines on the antibody and thiols on maytansinoid without additional linker structures.
Some sites surrounded by large antibody molecules ensure stability and efficient release of payloads without the need for adjacent substituent optimization.
Additionally, the use of carbamate-linked disulfide bonds in MMAE can efficiently release payloads and significantly extend the scope of small molecule payloads suitable for disulfide linkage.
Our Related ADC Target Proteins
Exogenous Induced Elimination
ADC drugs containing thioether bonds showed lower potency in HER2+ cell testing compared to doxorubicin itself, but the same potency was observed in the presence of the degradation reagent [Pd(COD)Cl2].
Enzymatic Cleavage Linkers
Specific enzymes in both cellular lysosomes and the extracellular tumor microenvironment selectively cleave corresponding substrates.
Cathepsin-Cleavable Dipeptides
Dipeptide-based linkers typically target cathepsin B, mainly found in lysosomes, with activity also observed extracellularly.
These dipeptides require a hydrophilic residue at the P1 position and a hydrophobic residue at the P2 position to be cleaved by proteases, maintaining linker stability during plasma transport.
Doxorubicin ADCs require spacer units like p-aminobenzyl carbamate (PABC) between dipeptides.
After dipeptide cleavage, PABC self-degrades quickly, releasing the original doxorubicin drug.
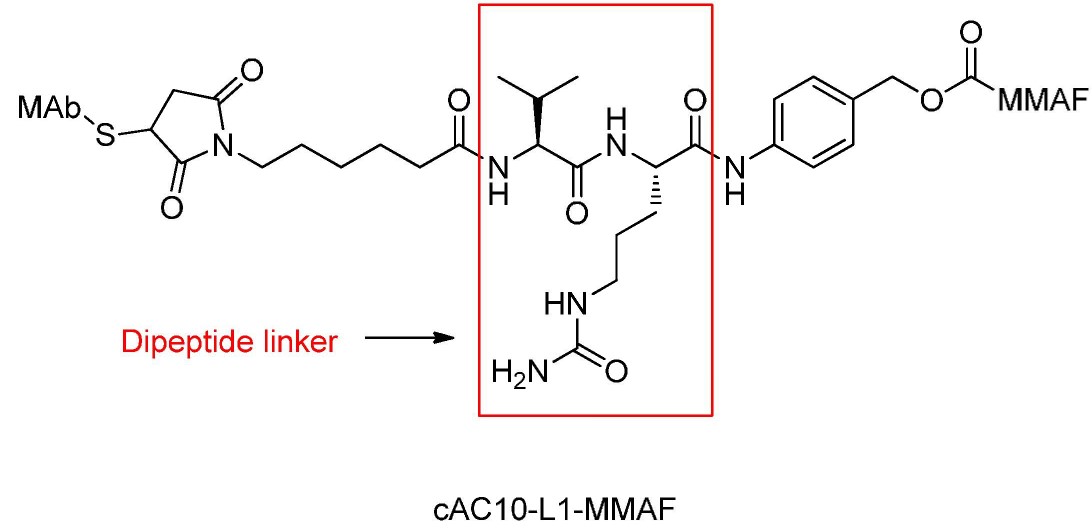
Glycosidase Cleavage
β-glucuronidase is a glycoside hydrolase that catalyzes the breakdown of β-glucuronic acid residues in polysaccharides.
PABC-containing glucuronic acid units have been researched, demonstrating greater plasma stability than dipeptides and effectively releasing original payloads.
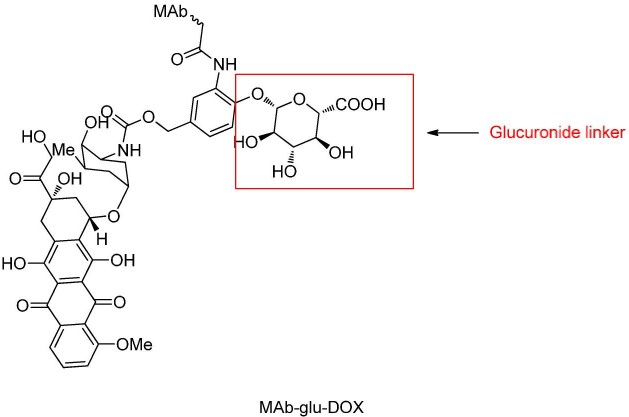
Glucuronic acid linkers also offer the advantage of high hydrophilicity, markedly reducing ADC aggregation, allowing the synthesis of high-load ADC drugs (DAR=8). β-galactosidase cleaves linkers similarly to glucuronidases.
Phosphatase Cleavage
Acid phosphatase and pyrophosphatase enzymes in lysosomes hydrolyze phosphoester bonds.
Merck designed a diphosphate linker to release prototype glucocorticoid payloads in lysosomes. Drugs linked with monophosphate linkers displayed relatively slower release.
Conclusion
Most ADCs currently utilize disulfide or dipeptide linkers for efficient differentiation between plasma and target cell conditions. Both techniques are now well-established and undergoing further development to better address solubility, plasma stability, and release functionality.
Related Products and Services
- ADC Target Protein
- PROTAC Targets
- Cell and Gene Therapy
- Targets of CAR-T Cell Therapy
- Cancer Drug Targets
- Immune Checkpoint Proteins
- Protein Engineering Services
- Protein Interaction Service
- Protein Expression and Purification Services
- Drug Discovery Screening
- Protein Pathway Profiling
Reference
- Lu, J., Jiang, F., Lu, A., & Zhang, G. (2016). Linkers Having a Crucial Role in Antibody–Drug Conjugates. International Journal of Molecular Sciences, 17(4), 561. https://doi.org/10.3390/ijms17040561

