Uncategorized Monday, 2025/01/20
Researchers from the University of California have published a research paper titled “FBP1 controls liver cancer evolution from senescent MASH hepatocyte” in the journal Nature.
The study resolved the contradiction between the state of aging cells and liver cancer, revealing the key role of FBP1 in regulating liver cell aging and HCC development, as well as the interaction between FBP1 and p53 and NRF2.
The results indicate that the loss of FBP1 promotes tumorigenesis through AKT activation, while the re-expression of FBP1 can inhibit the development of HCC.
The characteristics of metabolic dysfunction associated steatohepatitis (MASH) are lipid accumulation and inflammation, which cause liver cells to enter a tumor-suppressive state called senescence, but at the same time increase the risk of cancer. The team’s research on this paradox suggests that a diet that induces MASH triggers aging by causing DNA damage. However, prolonged metabolic stress causes these aging liver cells to begin dividing and progress into cancer.
Our Related Proteins
| Cat.No. # | Product Name | Source (Host) | Species | Tag | Protein Length | Price |
|---|---|---|---|---|---|---|
| AKT1-786H | Recombinant Full Length Human AKT1, His tagged | Insect Cells | Human | His | Full L. 1-480 aa | |
| TP53-58H |
Recombinant Human TP53, His-tagged

|
E.coli | Human | His | ||
| FBP1-389H |
Active Recombinant Human FBP1 Protein, His-tagged
|
E.coli | Human | His |
|
|
| AKT1-3920H | Recombinant Human AKT1 protein, GST-tagged | E.coli | Human | GST | 1-224aa | |
| TP53-15H |
Recombinant Human TP53 protein, GST-tagged

|
E.coli | Human | GST | ||
| GABPA-554H | Recombinant Human GABPA Protein, His-tagged | E.coli | Human | His | Ala168~Thr314 | |
| TP53-1162CAF488 |
Active Recombinant Cynomolgus TP53 Protein, Alexa Fluor 488 conjugated
|
E.coli | Monkey | 393 | ||
| TP53-1162CF |
Active Recombinant Cynomolgus TP53 Protein, FITC conjugated
|
E.coli | Monkey | 393 |
Previous studies have shown that liver cancer can be inhibited by the gluconeogenic enzyme fructose-1,6-diphosphatase-1 (FBP1). In this latest study, the research team found that FBP1 levels in human hepatocellular carcinoma (HCC) samples were lower than normal levels, consistent with a decrease in tumor suppressor protein TP53. The research team also observed the activation of the cancer-promoting enzyme AKT, while FBP1 contributed to its inactivation. It is known that AKT can accelerate the degradation of TP53 and stabilize the pro-cancer transcription activator protein NRF2. In addition, the research team also observed that in HCC, the FBP1 gene undergoes methylation modification, which prevents transcription activators (such as TP53) from promoting its expression.
Surprisingly, compared to the control group, FBP1, and TP53 levels were higher in human MASH samples and mouse models, while AKT and NRF2 levels were lower.
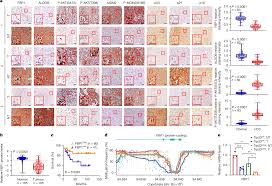
The liver cells of a mouse model induced by the MASH diet exhibit DNA damage. This triggers a DNA damage response, followed by activation of TP53, increased expression of FBP1, and an increase in molecular markers of aging. The research team investigated how liver cells with DNA damage evade aging and develop into hepatocellular carcinoma. They found that a group of cells called disease-related liver cells (daHep) exhibited activation of NRF2 and AKT, as well as relatively low levels of TP53 and FBP1.
These disease-related liver cells, similar to hepatocellular carcinoma progenitor cells, form tightly packed clusters of cells in the pre-cancerous liver. The presence of activated NRF2 in liver cells promotes the degradation of FBP1 and TP53, and enables cells to break free from aging. When mice are genetically engineered to lack FBP1, the development of HCC in mice carrying MASH or expressing the oncogenic mutant protein NRASG12V (which also damages DNA and induces aging) accelerates.
It is worth noting that MASH-induced aging relies on an enzyme that senses single-stranded DNA damage, while NRASG12V also activates another enzyme that senses double-stranded DNA damage. Interestingly, the mutations observed in MASH-induced HCC indicate the presence of single-stranded DNA damage, while the mutations observed in tumors induced by NRASG12V reflect both single-stranded and double-stranded DNA breaks. Cell lineage tracing shows that the majority of HCC originates from aging liver cells.
Based on the above findings, the research team concluded that although MASH-induced senescence reduces DNA damage in liver cells, it is not effective in inhibiting tumor formation. In fact, by preventing liver cell division caused by DNA damage, aging actually increases the risk of cancer because it promotes the survival of mutated liver cancer progenitor cells.
The aging state is unstable, and after long-term metabolic stress, disease-related liver cells (daHep) will re-enter the cell division cycle and develop into HCC. The research team further speculates that other cancers caused by DNA-damaged progenitor cells, whether or not they have FBP1 present only in the liver and kidneys, follow the same pathway.
In fact, many cancers develop from aging precancerous lesions (such as moles on the skin) that contain DNA-damaged cells that rapidly divide after escaping aging. Even if cancer cells express wild-type TP53 and have functional DNA damage responses, they may still enter an aging state when receiving DNA damage therapy. After treatment is stopped, these cells may produce offspring with many mutations, which are particularly invasive and difficult to eradicate.
For this study, the editor of the Nature journal stated that MASH is one of the fastest-growing obesity-related diseases globally and can further develop into liver cancer. In the past decade, there has been significant investment and research in this field, but this disease is still difficult to treat. This latest study reveals the interactions between liver metabolism, inflammation, aging, and cancer, deepening our understanding of the progression of MASH to liver cancer.
Related Products and Services
Protein Expression and Purification Services
Reference
Gu, L., Zhu, Y., Nandi, S. P., Lee, M., Watari, K., Bareng, B., Ohira, M., Liu, Y., Sakane, S., Carlessi, R., Sauceda, C., Dhar, D., Ganguly, S., Hosseini, M., Teneche, M. G., Adams, P. D., Gonzalez, D. J., Kisseleva, T., E., J. E., . . . Karin, M. (2024). FBP1 controls liver cancer evolution from senescent MASH hepatocytes. Nature, 637(8045), 461-469. https://doi.org/10.1038/s41586-024-08317-9
