Uncategorized Sunday, 2025/04/13
Why is it said that “middle-aged people find everything difficult except for gaining weight”? Research has found the culprit: a significant reduction in key anti-inflammatory molecules in the EVs secreted by middle-aged people’s fat cells, leading to metabolic disorders.
This study is the first to uncover why middle-aged people are more prone to obesity: as the adipose progenitor cells age, the extracellular vesicles (EVs) they secrete can no longer effectively convey anti-inflammatory signals (miR-145-5p), resulting in uncontrolled macrophages that cause adipose tissue inflammation and metabolic disorders.
As we age, our bodies undergo subtle yet significant changes. One widely mocked and troubling phenomenon is “middle-aged weight gain.” Data indicates that over 43% of people aged 30-49 globally face obesity issues, with all-cause mortality rates 22% higher in middle-aged obese individuals compared to their non-obese peers.
What’s more disheartening is that even when many middle-aged individuals maintain the same or even healthier eating and exercise habits as when they were younger, they are unable to avoid an expanding waistline and increasing weight. The traditional explanation often blames “slower metabolism with age,” but this vague excuse clearly doesn’t satisfy scientists’ curiosity—is there a neglected “traitor” hidden within middle-aged people’s fat cells?
Our related Proteins
| Cat.No. # | Product Name | Source (Host) | Species | Tag | Protein Length | Price |
|---|---|---|---|---|---|---|
| IL1B-02H |
Recombinant Human IL1B protein

|
E.coli | Human | Non | 153 | |
| IL6-12H |
Recombinant Human IL6 protein

|
E.coli | Human | Non | 183 | |
| Sell-2278M | Recombinant Mouse Sell protein, His-tagged | HEK293 | Mouse | His | 1-332 a.a. | |
| Il1b-383M | Active Recombinant Mouse Il1b protein | E.coli | Mouse | Non | Val118-Ser269 | |
| Il1b-384R | Recombinant Rat Il1b protein(Met1-Ser268), His-tagged | E.coli | Rat | His | Met1-Ser268 | |
| IL1B-744H | Active Recombinant Human IL1B protein(Met1-Ser269), His-tagged | E.coli | Human | His | Met1-Ser269 | |
| IL6-8849C | Recombinant Cynomolgus IL6, None tagged | E.coli | Cynomolgus | His | 29-212 a.a. | |
| IL1B-14167H | Recombinant Human IL1B protein, GST-tagged | E.coli | Human | GST | 1-269 aa | |
| IL1B-577R | Recombinant Rat IL1B, His tagged | E.coli | Rat | His | 117-268 a.a. | |
| TNFA-3314H | Recombinant Human TNFA, His-tagged | E.coli | Human | His | 1-233aa | |
| IL6-326 | Recombinant Cynomolgus Monkey IL6 protein | Yeast | Cynomolgus | Non | 185 aa | |
| Sell-831M |
Active Recombinant Mouse Sell Protein, His & Fc-tagged
|
HEK293 | Mouse | Fc&His | 1-332 a.a. |
|
| IL1B-1167C |
Active Recombinant Cynomolgus IL1B Protein
|
E.coli | Cynomolgus | Non | 117-269 a.a. | |
| IL1B-132H | Active Recombinant Human IL1B Protein | E.coli | Human | Non | 117-269 a.a. | |
| IL6-4372R | Recombinant Rabbit IL6 Protein | Yeast | Rabbit | Non | 188aa | |
| tnfa-4382Z | Recombinant Zebrafish tnfa Protein | Yeast | Zebrafish | Non | 148aa | |
| TNFa-4389A | Recombinant Atlantic Salmon TNFa Protein | Yeast | Atlantic Salmon | Non | 152aa | |
| IL6-040H | Recombinant Human IL6 Protein, non-tagged, Biotinylated | HEK293 | Human | Non | 30-212 a.a. | |
| mouse Tnfa-2639H | Recombinant Mouse Tnfa protein, His-tagged | E.coli | Mouse | His | 1-235 aa | |
| SELL-2810H | Recombinant Human SELL protein, His-tagged | E.coli | Human | His | 233-375 aa | |
| IL6-02H | Active Recombinant Human IL6 Protein (30-212aa), N-His-tagged | E.coli | Human | His | 30-212aa |
A recent study published in Nature Communications provides an answer: the “couriers” in adipose tissue—extracellular vesicles (EVs) secreted by adipose progenitor cells (APCs)—are the “invisible influencers” of middle-aged obesity! The research found that the EVs secreted by middle-aged people’s APCs experience a sharp decline in the key anti-inflammatory molecule miR-145-5p, leading to chronic inflammation of adipose tissue (commonly known as “inflammaging”) and ultimately causing metabolic disorders. Interestingly, the team also designed targeted liposomes that successfully delivered miR-145 to adipose macrophages, reversing obesity in middle-aged mice! This study not only opens the molecular black box of middle-aged weight gain but also provides a new target for anti-obesity drug development.
What exactly are extracellular vesicles?
In the enormous “cellular city” of the human body, cells are not isolated; they are constantly in contact with each other. In addition to classic hormone and nerve conduction methods, extracellular vesicles (EVs) have emerged in recent years as new “communication tools” gaining significant attention in biomedical research.
EVs are nanoscale membrane vesicles released by cells through exocytosis, typically ranging from 30-150 nanometers in diameter. Like “delivery vehicles,” they carry various biomolecules such as proteins, lipids, mRNA, and microRNA (miRNA) between cells, delivering “instructions” precisely to their destinations. These EVs can regulate inflammation, metabolism, immune responses, and other key processes, playing the role of “behind-the-scenes orchestrators” in the development of obesity, diabetes, cardiovascular diseases, and even tumors.
High-fat diet experiments reveal: middle-aged mice gain weight more easily than young mice.
To verify whether there is a clear physiological basis for “middle-aged weight gain,” the research team used an age-matched mouse model: 3-month-old mice represented “young people,” while 12-month-old mice represented “middle-aged people.”
Both groups of mice were fed a high-fat diet (HFD) for four weeks. The results showed that the middle-aged mice experienced significantly faster weight gain and exhibited more severe glucose tolerance abnormalities and insulin resistance. Flow cytometry further revealed a noticeable increase in the number of pro-inflammatory M1 macrophages in their adipose tissue, accompanied by upregulated inflammatory factors such as TNF-α, IL-1β, and IL-6.
By comparing transcriptome changes across multiple metabolic organs (such as the liver, skeletal muscle, and white adipose tissue) of the mice, the study found that significant transcriptome reprogramming occurred only in white adipose tissue (WAT) in middle-aged mice, with a much greater number of differentially expressed genes compared to other organs, suggesting that WAT might be the “first stop” leading to middle-aged obesity.
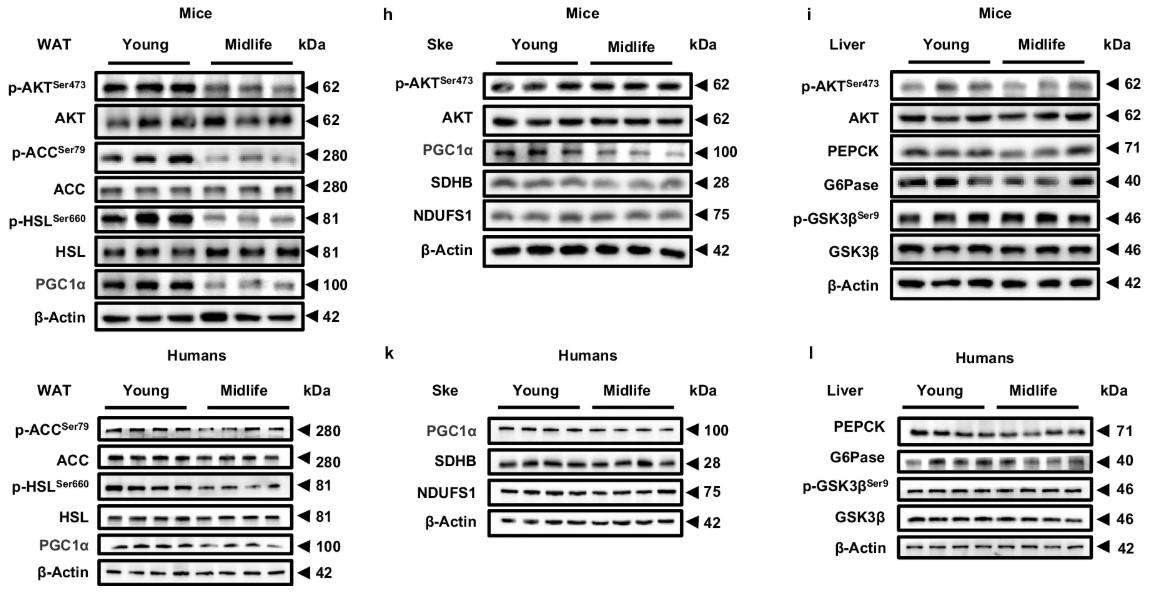 Fig2. Western blot analysis of p-AKTSer473/AKT, p-ACCSer79/ACC, p-HSLSer660/HSL and PGC1α in WAT
Fig2. Western blot analysis of p-AKTSer473/AKT, p-ACCSer79/ACC, p-HSLSer660/HSL and PGC1α in WATAging adipose progenitor cells result in “failed deliveries.”
So, which cell subtype in WAT is “to blame”?
The research team conducted single-nucleus RNA sequencing (snRNA-seq) on adipose tissue from young and middle-aged individuals, identifying 25 cell subtypes. Among them, APCs—responsible for adipose tissue renewal and differentiation—exhibited the most pronounced aging features in middle-aged samples. Not only did these cells decrease in number, but they also showed significant signs of aging (such as upregulation of genes like p16, p21, and p53), and their secreted EVs underwent notable changes.
Further analysis found that the critical anti-inflammatory miRNA in EVs secreted by middle-aged APCs, miR-145-5p, experienced a sharp reduction compared to those from younger individuals.
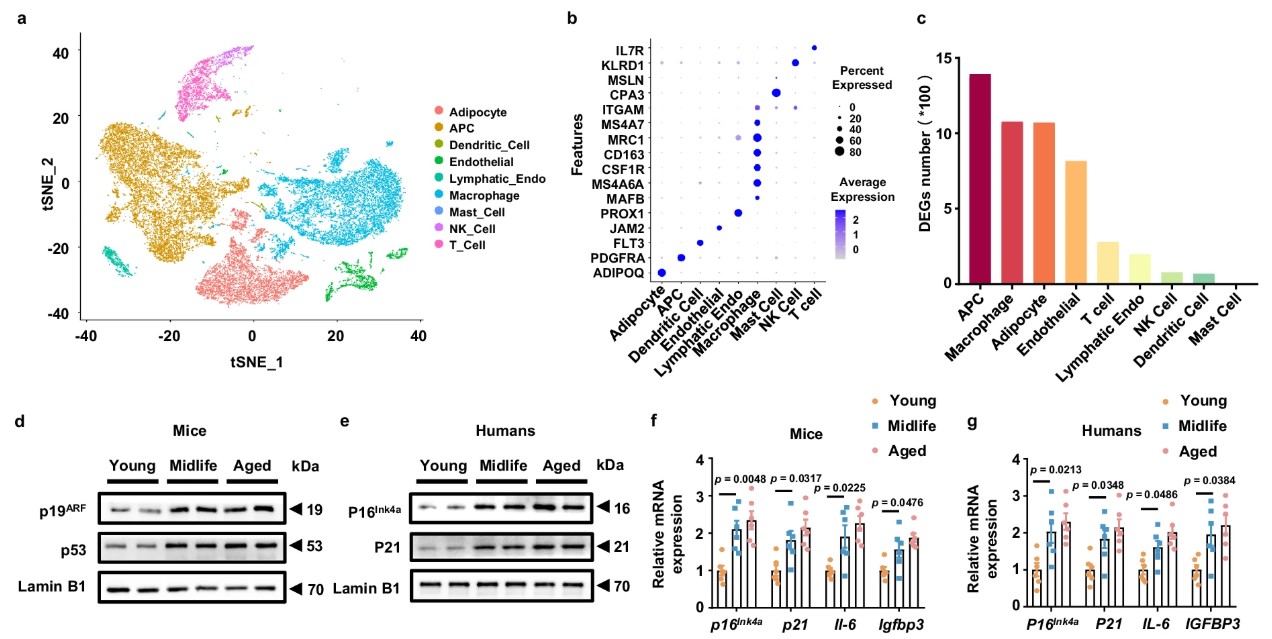 Fig3. Midlife APC-derived EVs exhibit a reduced capacity to maintain adipose immune homeostasis.
Fig3. Midlife APC-derived EVs exhibit a reduced capacity to maintain adipose immune homeostasis.EVs lacking miR-145 fail to suppress “rampant macrophages.”
Macrophages are the “immune sentinels” in adipose tissue, and they exhibit two “personalities”—M1 type with a “flaring temper” responsible for clearing infections but prone to causing chronic inflammation; M2 type that is mild and reparative, maintaining tissue homeostasis.
Experiments showed that EVs released by young APCs can be engulfed by macrophages, delivering miR-145-5p effectively, inhibiting the activation of M1 macrophages and pro-inflammatory factor expression. However, due to the insufficient miR-145 content in EVs from middle-aged APCs, control over macrophages diminishes, leading to uncontrolled M1 macrophages, chronic low-grade inflammation (inflammaging) of adipose tissue and eventually causing middle-aged obesity and metabolic disorders.
Mechanistic studies further revealed that miR-145’s key target is SELL (L-selectin), a molecule involved in cell adhesion and migration whose upregulation can activate the NF-κB signaling pathway, driving the polarization of macrophages to the M1 type. By directly inhibiting SELL, miR-145 in EVs regulates immune balance and mitigates inflammation.
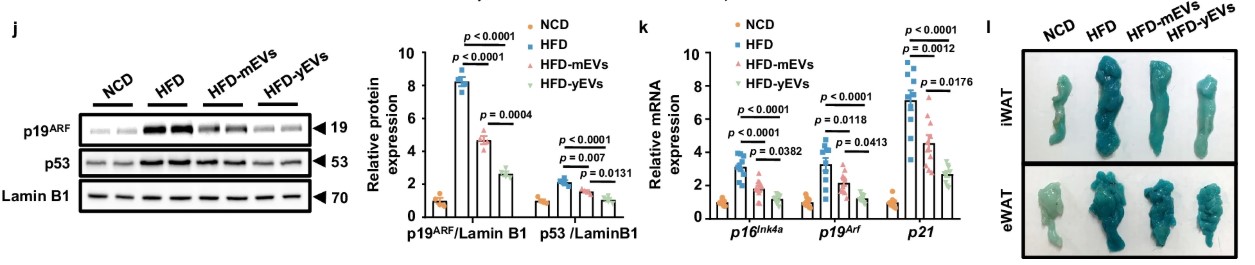 Fig4. APC-derived EVs suppress WAT inflammaging to prevent obesity
Fig4. APC-derived EVs suppress WAT inflammaging to prevent obesity“Supplementary delivery” experiment: delivering miR-145 could lead to weight loss!
If the issue lies in the lack of miR-145 delivery, can it be “supplemented”?
Researchers designed a cationic liposome targeted at macrophages (miR-145@tar-lip), mimicking the EVs’ function, carrying miR-145 specifically. They delivered it to middle-aged mice through tail vein injection.
The results were exciting! Middle-aged mice treated with miR-145 liposomes showed significantly slower weight gain under a high-fat diet, reduced inflammation in adipose tissue, improved insulin sensitivity, and effectively restored metabolic function. More importantly, these liposomes accurately delivered to macrophages within adipose tissue, exercising targeted effects with almost no side effects!
Summary
This study reveals why middle-aged people are more prone to obesity: as adipose progenitor cells age, their secreted extracellular vesicles (EVs) fail to effectively convey anti-inflammatory signals (miR-145-5p), leading to uncontrolled macrophages, adipose tissue inflammation, and metabolic disorders.
This implies that middle-aged obesity is not simply due to “eating more, moving less,” but is a result of profound cellular communication breakdowns.
Notably, miR-145 not only regulates inflammation and obesity but also plays multiple roles in cardiovascular diseases, tumors, and even neurodegenerative diseases. Therefore, using EVs as “carriers” and miRNAs as “drugs” could have applications in multiple fields.
Next steps for scientists might include developing oral or subcutaneous forms of EV mimics to lower treatment thresholds; they may also engineer and customize EVs for personalized metabolic interventions. In any case, future weight loss drugs might no longer depend on appetite suppression or metabolic acceleration but on restoring “Smooth communication between cells.” Doesn’t that sound incredible! Let’s look forward to it together!
Related Products & Services
- Obesity Targets
- Cell and Gene Therapy
- Immune Checkpoint Proteins
- Protein Engineering Services
- Protein Interaction Service
- Protein Expression and Purification Services
- Drug Discovery Screening
- Protein Pathway Profiling
Reference
- Kumar, M.A., Baba, S.K., Sadida, H.Q. et al. Extracellular vesicles as tools and targets in therapy for diseases. Sig Transduct Target Ther 9, 27 (2024). https://doi.org/10.1038/s41392-024-01735-1
- Zhou, Q., Gao, J., Wu, G., Wang, C., Yang, Y., Huang, T., Wang, Y., Yue, T., Gao, Z., Xie, H., Xiong, F., Xiang, K., Yong, T., Zhang, W., Zhang, T., Kong, W., Chen, C., Zhang, S., Yu, Q., Fan, X., … Wang, C. Y. (2025). Adipose progenitor cell-derived extracellular vesicles suppress macrophage M1 program to alleviate midlife obesity. Nature communications, 16(1), 2743. https://doi.org/10.1038/s41467-025-57444-y
