Uncategorized Thursday, 2024/10/17
The immune system needs to control the immune response to maintain a balance between inhibiting infection and preventing excessive inflammation from damaging tissues. As the most numerous type of immune cells in the circulatory system, neutrophils play an important defense role against a large number of pathogens. At the same time, to maintain the balance of immune response, the antimicrobial response of neutrophils must be accurately regulated. Neutrophils are activated by pro-inflammatory signals and activate different effector effects based on the composition of surface receptors and endogenous proteins. For example, neutrophils engulf microorganisms and produce reactive oxygen species, undergo degranulation, or release NETs (Neutrophil extracellular traps).
NETs are extracellular structures mainly composed of DNA, granule proteins, nucleoproteins, etc., which are assembled on decondensed chromatin scaffolds. NETs can encapsulate, neutralize, and kill microorganisms, including fungi, bacteria, and parasites. However, other stimuli such as crystals and immune complexes can also induce the formation of NETs. It is worth noting that in some autoimmune diseases, neutrophils are activated and release NETs, such as rheumatoid arthritis (RA) and systemic lupus erythematosus (SLE). NETs contain many molecules that can be recognized by receptors of immune cells. For example, after macrophages and dendritic cells engulf NETs, their intracellular cGAS (cyclic GMP AMP synthase) can recognize the DNA backbone in NETs. In addition, the cell surface receptor TLR9 can also recognize NET-DNA. Some studies have also found that NETs can activate membrane receptors of some immune cells, but their mechanism of action has not been fully understood.
Recently, Gordon D. Brown's team from the University of Exeter in the UK published an article in Nature titled "Recognition and control of neutrophil extracellular trap formation by MICL". They found that the absence of the receptor MICL (myoid inhibitory C-type lectin-like) can promote the progression of rheumatoid arthritis by promoting the formation of neutrophil NETs.
| Cat.No. # | Product Name | Source (Host) | Species | Tag | Protein Length | Price |
|---|---|---|---|---|---|---|
| NOX1-1335H | Recombinant Human NOX1, His-tagged | E.coli | Human | His | 292-560aa | |
| TLR9-48H |
Recombinant Human TLR9 protein

|
E.coli | Human | Non | ||
| NOX1-1359H | Recombinant Human NOX1 protein, His-tagged | E.coli | Human | His | Met235~Asn488 | |
| PADI4-313H | Recombinant Human PADI4 Protein, His-tagged | E.coli | Human | His | Met1~Pro300 | |
| CLEC12A-213H |
Active Recombinant Human CLEC12A Protein, hFc-tagged
|
HEK293 | Human | Fc | His65-Ala265 |
|
| CLEC12A-2947H |
Active Recombinant Human CLEC12A protein, Fc-Avi-tagged, Biotinylated
|
HEK293 | Human | Avi&Fc | His 65-Ala 265 |
|
| CLEC12A-2948H |
Recombinant Human CLEC12A protein, His,Avi-tagged, Biotinylated
|
HEK293 | Human | Avi&His | His65-Ala265 |
|
| CLEC12A-2950H |
Active Recombinant Human CLEC12A protein, Fc-tagged
|
HEK293 | Human | Fc | His 65-Ala 265 |
|
| CLEC12A-20H |
Active Recombinant Human CLEC12A Protein (Thr67-Ala265), N-hFc tagged
|
CHO | Human | Fc | Thr67-Ala265 |
|
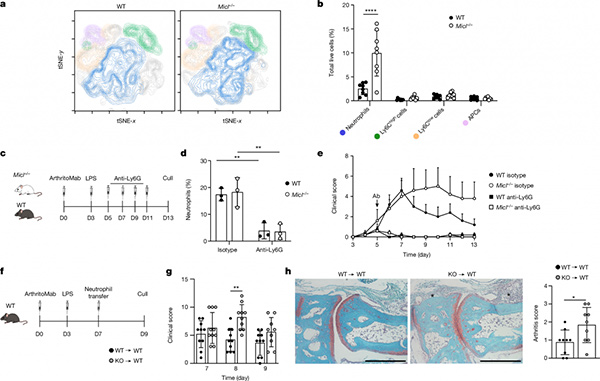
Researchers used Micl -/- mice to construct a CAIA (collagen antibody-induced arthritis) model of rheumatoid arthritis, and Micl knockout mice showed enhanced and non-relieved arthritis phenotypes. Researchers analyzed the joint tissues of wild mice on the 7th day of modeling, which showed the most significant disease progression. Through flow cytometry analysis, it was found that the infiltration of neutrophils in joint tissues specifically increased in Micl -/- mice. The number of other cell types in the joint tissue did not change, and the number of neutrophils in the bone marrow and blood also did not change. This result was also obtained in another K/BxN model of RA.
In order to investigate the role of neutrophils in RA progression and their relationship with MICL, researchers applied anti-Ly6G to clear circulating neutrophils in the CAIA model. They found that disease progression in wild-type mice and Micl -/- mice decreased to the same level, indicating that the RA progression caused by Micl knockout is achieved through neutrophils. Furthermore, researchers isolated neutrophils from the bone marrow of WT and Micl -/- mice and then transplanted these neutrophils into RA model mice on the 7th day of the CAIA model. The researchers observed that neutrophils from Micl -/- mice exacerbated arthritis. This result further confirms the crucial role of neutrophils in the progression of RA in Micl -/- mice.
Next, researchers found that neutrophils derived from Micl -/- mice had increased ROS levels under stimulation with monosodium urate (MSU) or A. fumigatus hypha. As NET formation is related to ROS levels, researchers detected that Micl deficiency leads to corresponding enhancement of NET formation. The NADPH oxidase inhibitor DPI (diphenyl iodonium chloride) inhibited the formation of NET, indicating that the formation of NET caused by Micl deficiency depends on the activity of NADPH oxidase. In addition to ROS, the formation of NET also requires histone citrullination modification, which depends on the function of the PAD4 protein. PAD4 inhibitors can inhibit the formation of NETs in vitro experiments, and in vivo experiments have also shown that PAD4 inhibitors significantly reduce the number of NETs in the joints of Micl -/- mice, but do not affect the infiltration of neutrophils in the joints.
Previous studies by researchers have found that monoclonal antibodies against MICL can exacerbate the pathological degree of wild-type mouse CAIA models, similar to the phenotype of Micl -/- mice. In vitro experiments also showed that MICL monoclonal antibodies induced an increase in MSU-mediated ROS production in neutrophils. In addition, the stimulating effect of MICL monoclonal antibody on the pathology of CAIA model mice can be inhibited by PAD4 inhibitors. This has been validated in other animal models of RA such as CIA (collaboration-induced arthritis). Researchers further discovered that human MICL antibodies can promote the activation of neutrophils and the formation of NETs. Serum from RA patients can also promote the production of ROS, indicating that the MICL antibodies contained in RA patients' serum inhibit the function of MICL, and promote the activation of neutrophils and the formation of NET.
So, the researchers further examined the relationship between the levels of MICL antibodies in the serum of RA patients and the severity of RA pathology, and found that the overall level of MICL antibodies in RA patients was higher than that in the control group. In addition, in the serum of patients with NET-related diseases such as systemic lupus erythematosus and COVID-19, the antibody levels against MICL are also higher than those in normal individuals, indicating that the activation of neutrophils after MICL inhibition is closely related to the progression of these immune diseases.
Finally, researchers found that MICL is a PRR receptor that can recognize genomic DNA in NET, and this recognition can be disrupted by MICL antibodies.
In summary, this study found that the receptor MICL can regulate the activation of neutrophils in autoimmune diseases. Some autoimmune disease patients produce excessive MICL antibodies, which inhibit the function of MICL and lead to sustained activation of neutrophils, disrupting the immune balance of the body. This study confirms the key molecular mechanisms for maintaining immune balance in autoimmune diseases, providing new ideas and targets for the treatment of related diseases.
Related Products and Services
Immune Checkpoint Proteins
Protein Engineering Services
Protein Interaction Service
Protein Expression and Purification Services
Drug Discovery Screening
Protein Pathway Profiling
Reference
Malamud M, Whitehead L, McIntosh A, et al. Recognition and control of neutrophil extracellular trap formation by MICL. Nature. 2024 Sep;633(8029):442-450. doi: 10.1038/s41586-024-07820-3. Epub 2024 Aug 14. PMID: 39143217.
