Uncategorized Monday, 2025/01/20
Cancer can trigger multiple pathways to slow down external and internal stress, thereby continuing its growth and progression. Previous studies have reported that eIF2 kinase GCN2 and integrated stress response have certain constitutive activity in prostate cancer and require maintenance of the amino acid homeostasis required to promote tumor growth. However, although the loss of GCN2 function reduces the availability of intracellular amino acids and the growth of prostate cancer, there is no significant cell death.
Recently, a research report titled “Coordination between the eIF2 kinase GCN2 and p53 signaling supports purine metabolism and the progression of prostate cancer” was published in the journal Science Signaling. Scientists from Indiana University School of Medicine and other institutions have revealed a new weakness in animal models of prostate cancer, which may cause prostate tumors to lack key nutrients and hinder their growth. This may help develop new therapies for this deadly disease.
Our Related Proteins
| Cat.No. # | Product Name | Source (Host) | Species | Tag | Protein Length | Price |
|---|---|---|---|---|---|---|
| EIF2C1-2196H | Recombinant Human EIF2C1, His tagged | Insect Cells | Human | His | Full L. 1-857 a.a. | |
| Eif2c2-4071M | Recombinant Mouse Eif2c2, His tagged | Insect Cells | Mouse | His | 1-860 a.a. | |
| EIF2AK4-1011H | Recombinant Human Eukaryotic Translation Initiation Factor 2 Alpha Kinase 4, GST-tagged | Sf9 Cells | Human | GST | 192-1024 a.a. |
|
| EIF2AK2-12350H | Recombinant Human EIF2AK2, His-tagged | E.coli | Human | His | 1-373a.a. | |
| EIF2B2-12354H | Recombinant Human EIF2B2, GST-tagged | E.coli | Human | GST | 1-351a.a. | |
| EIF2C2-2180H |
Active Recombinant Human EIF2C2, His-tagged

|
Insect Cells | Human | His | Met 1-Ala 859 | |
| EIF2AK4-5078M | Recombinant Mouse EIF2AK4 Protein | Mammalian Cells | Mouse | His |
|
|
| EIF2AK2-3148H |
Active Recombinant Human EIF2AK2 Protein, GST-tagged
|
Insect Cells | Human | GST | 252-551 a.a. | |
| EIF2AK4-3346H | Recombinant Human EIF2AK4 protein, His-tagged | E.coli | Human | His |
Prostate cancer is one of the leading causes of cancer death in American men, and current therapies can target the hormone testosterone, which is essential for the growth of prostate cancer cells. Unfortunately, prostate tumors frequently develop tolerance to these therapies, leaving clinicians with almost no available means to block this disease. In this study, researchers have discovered a promising new approach that can target prostate cancer by depriving it of key nutrients called amino acids. Like other tumors, prostate cancer cells require a large amount of nutrients to support their rapid growth. As the nutrients are depleted, a protein called GCN2 sends signals to the cells to produce more fuel to support growth. Therefore, researchers speculate that a drug that can turn off GCN2 may prevent cancer from producing enough fuel to survive.
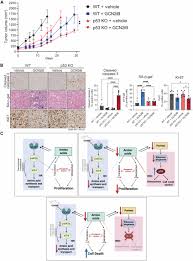
Professor Staschke, a researcher, said that we may only be partially correct. Inhibiting GCN2 can indeed slow down the growth of tumor cells, but it cannot kill them. It was then that we discovered that cancer cells may have a backup plan. So through further research, we found that the protein called p53 may be the “backup plan” for cancer. Unlike other forms of cancer, p53 retains its function in most prostate tissues, sending signals to restrict cell division and collect nutrients. When researchers inhibit GCN2 and p53, prostate cancer may be effectively destroyed.
Current research has also exploited the metabolic weaknesses unique to prostate cancer, resulting in a lack of essential nutrients and the ability to kill these tumor cells. In summary, this study emphasizes the coordinated interaction between GCN2 and p53 regulation during nutritional stress, and provides new research insights into how their interaction is targeted to develop novel therapies for human prostate cancer.
Related Products and Services
Protein Expression and Purification Services
Reference
RICARDO A. CORDOVA,NOAH R. SOMMERS,ANDREW S. LAW, et al. Coordination between the eIF2 kinase GCN2 and p53 signaling supports purine metabolism and the progression of prostate cancer, Science Signaling (2024). DOI:10.1126/scisignal.adp1375
