Uncategorized Friday, 2025/03/14
Recently, Nicolai Franzmeier's team from the Stroke and Dementia Research Institute at the University of Munich in Germany published an important research result. They found that the overactivity and enhanced functional connectivity of neurons induced by Aβ pathology are key factors in promoting the spread of tau pathology.
Specifically, the study first found that Aβ deposition enhances the functional connectivity between the pathological initiation core area of tau (lower temporal lobe) and the pathological susceptibility brain areas of tau (such as parietal and occipital lobes). Subsequently, in Aβ - positive patients, researchers observed that brain regions with stronger connections to the pathological initiation core area of tau often exhibited faster tau protein accumulation rates. The final mediation analysis further confirms that the effect of Aβ on tau accumulation is indirectly achieved by enhancing the connection between the pathological initiation core area and the susceptible brain area. The connection enhancement induced by Aβ can explain 5% to 25% of the tau protein accumulation effect.
The study was published in Science Translational Medicine.
Our Related Products
| Cat.No. # | Product Name | Source (Host) | Species | Tag | Protein Length | Price |
|---|---|---|---|---|---|---|
| MAPT-123H | Recombinant Human Tau-441 (G272V) | E.coli | Human | Non |
|
|
| MAPT-134H | Recombinant Human Tau-441 (dN296) | E.coli | Human | Non |
|
|
| MAPT-141H | Recombinant Human Tau-441 (S199E) | E.coli | Human | Non |
|
|
| MAPT-145H | Recombinant Human Tau-441 (S404A) | E.coli | Human | Non |
|
|
| MAPT-159H | Recombinant Human Tau-441 (V337M) | E.coli | Human | Non |
|
|
| TAU-301315H | Recombinant Human TAU protein, GST-tagged | E.coli | Human | GST | Pro4-Leu208 |
|
| TAU-301451H | Recombinant Human TAU protein, GST-tagged | E.coli | Human | GST | Ala87-Arg172 |
|
| Tau-441-2213H | Recombinant Human Tau-441 protein, His-tagged | E.coli | Human | His | Ser 241 - Glu 380 |
|
| tau-38D | Recombinant Drosophila melanogaster tau Protein (Full length) | E.coli | Drosophila melanogaster | Full L. Full Length |
|
|
| Tau dGAE-01H | Recombinant Full Length Human wild-type Tau 2N4R | E.coli | Human | Non | Full L. 297-391 aa |
|
Given that previous research has shown that Aβ can trigger neuronal overactivity and enhanced functional connectivity, while tau protein spreads between connected neurons in a manner similar to activity. Therefore, based on previous research results, researchers proposed a hypothesis that the overactivity and enhanced functional connectivity of neurons induced by Aβ are the key mechanisms by which Aβ pathology promotes the spread of tau pathology.
To validate this hypothesis, the researchers included 209 participants from the Alzheimer's Disease Neuroimaging Initiative (ADNI) database, including 69 healthy control participants with normal Aβ - negative cognition and 140 Aβ - positive participants (covering populations from preclinical to dementia stages, including cognitively normal, mild cognitive impairment, and AD patients), and analyzed their Aβ - PET, resting state functional magnetic resonance imaging (fMRI, primarily assessing functional connectivity and activity patterns between brain regions), and longitudinal tau - PET detection data.
As a result, it was found that Aβ deposition enhanced the functional connectivity between the tau pathological initiation core area (defined as the 5% brain area with the highest baseline tau PET SUVR values in Aβ positive individuals, specifically located in the lower temporal lobe) and tau pathological susceptibility brain areas (posterior brain areas such as the parietal and occipital lobes).
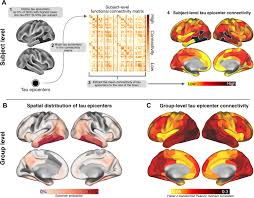
Subsequently, this phenomenon was also validated in an independent cohort consisting of 55 healthy control participants and 345 Aβ - positive participants. More importantly, further research has found that the influence of Aβ is not limited to a specific region, but is associated with enhanced functional connectivity across the entire brain, and this connectivity enhancement occurs in the early stages of AD.
The subsequent ROI regression analysis showed that brain regions with stronger functional connections to the pathological initiation core area of tau tend to exhibit faster tau protein accumulation rates, especially in the parietal, frontal, and occipital regions. This indicates that the propagation speed of tau is closely related to the strength of functional connections, and this result also supports the “cross neuronal propagation” theory of tau protein.
Finally, using mediation analysis, the researchers observed that the effect of Aβ on tau accumulation is indirectly achieved by enhancing the connectivity between the pathological initiation core area and susceptible brain areas, where Aβ - induced connectivity enhancement can explain 5% to 25% of the tau protein accumulation effect.
In summary, this study revealed that the neuronal overactivity and functional connectivity enhancement induced by Aβ pathology are key mechanisms promoting the spread of tau pathology. This mechanism not only fills the gap in the relationship between Aβ pathology and tau pathology, but also provides a new direction for the treatment of AD (such as slowing down the spread of tau protein by regulating neuronal activity or functional connections).
Related Products and Services
Neurological Disorders Targets Adhesion Molecules
Blood-brain Barrier Permeability
Calcium-binding Proteins and Related Molecules
Neurodegenerative Disease
Neurotransmitter Associated Enzymes
Neurotransmitter Receptors, Transporters, and Ion Channels
Neurotrophic Factors and Receptors
Synaptic Proteins and Receptors
Protein Engineering Services
Protein Interaction Service
Protein Expression and Purification Services
Drug Discovery Screening
Protein Pathway Profiling
Reference
Roemer-Cassiano SN, Wagner F, Evangelista L, Rauchmann BS, Dehsarvi A, Steward A, Dewenter A, Biel D, Zhu Z, Pescoller J, Gross M, Perneczky R, Malpetti M, Ewers M, Schöll M, Dichgans M, Höglinger GU, Brendel M, Jäkel S, Franzmeier N. Amyloid-associated hyperconnectivity drives tau spread across connected brain regions in Alzheimer's disease. Sci Transl Med. 2025 Jan 22;17(782):eadp2564. doi: 10.1126/scitranslmed.adp2564. Epub 2025 Jan 22. PMID: 39841807.
