Uncategorized Monday, 2024/07/01
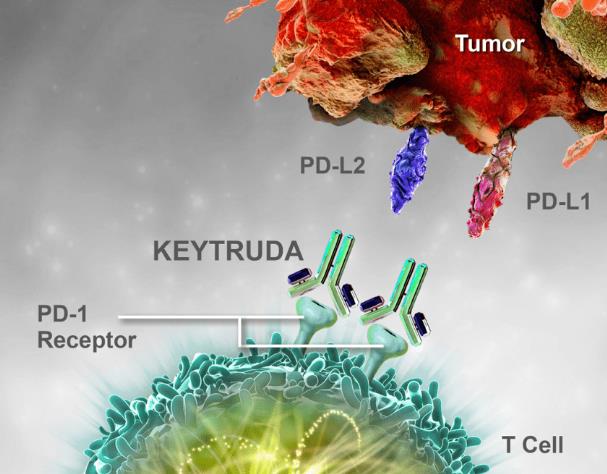
Keytruda is a PD-1 inhibitor developed by Merck, which can block the interaction between PD-1 and its ligands PD-L1 and PD-L2, release immune suppression mediated by the PD-1 signaling pathway, activate T lymphocytes that may affect tumor cells and healthy cells, and enhance the human immune system's ability to detect and eliminate cancer cells.
Since obtaining FDA approval for the treatment of advanced melanoma for the first time in 2014, Keytruda has received FDA approval for the treatment of at least 16 types of cancer, as well as indications for unlimited cancer types.
Our Featured Products
On June 18, 2024, the FDA approved the combination of Pembrolizumab, an MSD PD-1 inhibitor, with carboplatin and paclitaxel, followed by monotherapy with Keytruda for adult patients with primary advanced or recurrent endometrial cancer.
It is reported that Keytruda is the first PD-1 targeted therapy approved by the US FDA in combination with chemotherapy for the treatment of adult patients with primary advanced or recurrent endometrial cancer, regardless of the patient's mismatch repair (MMR) status.
This approval is mainly based on a phase 3 clinical trial, which included a total of 810 patients with advanced or recurrent endometrial cancer in this multicenter, randomized, double-blind, placebo-controlled trial, including two independent cohorts based on mismatch repair status: 222 patients in the mismatch repair defect (dMMR) cohort and 588 patients in the mismatch repair perfect (pMMR) cohort. Patients were randomly assigned to receive Keytruda combined chemotherapy (carboplatin and paclitaxel) followed by Keytruda monotherapy in a 1:1 ratio or to receive placebo combined chemotherapy followed by placebo monotherapy.
The main efficacy endpoint of the trial is progression-free survival (PFS). In the dMMR cohort, the Keytruda combination therapy group did not achieve a median PFS, while the placebo group had a median PFS of 6.5 months, with a significant statistical difference between the two. In the pMMR cohort, the median PFS of the Keytruda combination therapy group was 11.1 months, while the placebo group was 8.5 months, with a statistically significant difference between the two. The research results were published in the New England Journal of Medicine.
In the trial, the adverse reactions related to treatment in the Keytruda combination therapy group are usually similar to previously reported trial results. Still, the incidence of rash in patients is higher.
Related Products and Services
CD Antigens Targets of CAR-T Cell Therapy Cytokines Cancer Drug Targets Immune Checkpoint Proteins Protein Interaction Service Protein Expression and Purification Services Drug Discovery Screening Protein Pathway Profiling
