Uncategorized Tuesday, 2024/06/11
More than half of the approximately 5000 membrane proteins synthesized on the endoplasmic reticulum membrane of human cells are multi-transmembrane proteins. Multiple transmembrane proteins play important roles in cells as ion channels, transporters, receptor proteins, transmembrane enzymes, and more. These functions rely heavily on the polarity and charged amino acids of the transmembrane domain, and the polar and charged amino acid side chains typically exhibit lipid-repellent properties, resulting in lower hydrophobicity of the transmembrane helix (TMH) they are located in. Statistics show that approximately 30% of membrane proteins and over 50% of multiple transmembrane proteins in the human proteome contain at least one extremely low hydrophobicity TMH (pTMH). In the mature structure of multiple transmembrane proteins, pTMH is usually protected by surrounding TMH to avoid direct contact with the membrane structure of phospholipid bilayers. However, pTMH is often difficult to be directly recognized and inserted by transposons. How these pTMHs can be recognized and overcome the hydrophobicity of phospholipid environments, and how they can be packaged into mature multi-transmembrane structures are scientific issues that have not been fully understood in the relevant field.
Recently, Zhang Zairong's team from the Biology and Chemistry Cross Research Center of the Chinese Academy of Sciences Shanghai Institute of Organic Chemistry published a research paper entitled “AnATP13A1-assisted topogenesis pathway for folding multi-spanning membrane proteins” online on Molecular Cell, revealing the multiple transmembrane protein topology generation pathways guided by pTMH. Newborn pTMH cannot be immediately incorporated into the endoplasmic reticulum membrane, but instead enters the water-soluble endoplasmic reticulum cavity through the central pore of the translocator, causing downstream TMHs to be incorporated into the endoplasmic reticulum membrane in an incorrect direction opposite to the final structure. After synthesis, P5-ATPaseATP13A1 can recognize and correct the "wrong" intermediate configuration, making the pTMH trapped in the endoplasmic reticulum cavity recognizable and integrating and folding into the nearly mature structure, ultimately obtaining a mature conformation.
Our Featured Products
| Cat. No. | Product Name | Species | Source | Tag |
| ATP13A1-10000H | Recombinant Human ATP13A1, GST-tagged | Human | E.coli | GST |
| ATP13A1-845M | Recombinant Mouse ATP13A1 Protein, His (Fc)-Avi-tagged | Mouse | HEK293 | His (Fc)-Avi |
| ABCG2-067H | Recombinant Human ABCG2 Protein, GST-Tagged | Human | Wheat Germ | GST |
| ABCG2-659H | Recombinant Human ABCG2 | Human | Mammalian Cell | His |
| ABCG2-5672H | Recombinant Human ABCG2 protein, His-tagged | Human | E.coli | |
| ABCG2-2404H | Recombinant Human ABCG2 Protein, His (Fc)-Avi-tagged | Human | HEK293 | His (Fc)-Avi |
Using the six transmembrane transporter protein ABCG2 and human cells as experimental models, researchers found that the newly generated pTMH2 of ABCG2 can directly enter the endoplasmic reticulum cavity through the transposon, resulting in downstream TMHs that incorrectly integrate into the intermediate configuration state of the membrane. After the translation of the double lysine located at the carboxyl end is completed, the configuration of ABCG2 undergoes an almost global topological rearrangement process. Further research has found that ATP13A1 can perceive this positive double lysine signal. When the double lysine is mutated into a negative or electrically neutral amino acid, the interaction between ATP13A1 and ABCG2 mutants is significantly weakened compared to the wild-type. Knockout of ATP13A1 leads to the accumulation of ABCG2 in a folding intermediate state within cells. ATP13A1 can play a role in the topological structure maturation of multiple transmembrane proteins, promoting the dissociation of TMH6 reverse inserted in ABCG2 from the phospholipid bilayer. TMH6 exposed in the cytoplasm reinserts into the endoplasmic reticulum membrane in the correct orientation, thereby driving the post-translational topological rearrangement of upstream TMHs.
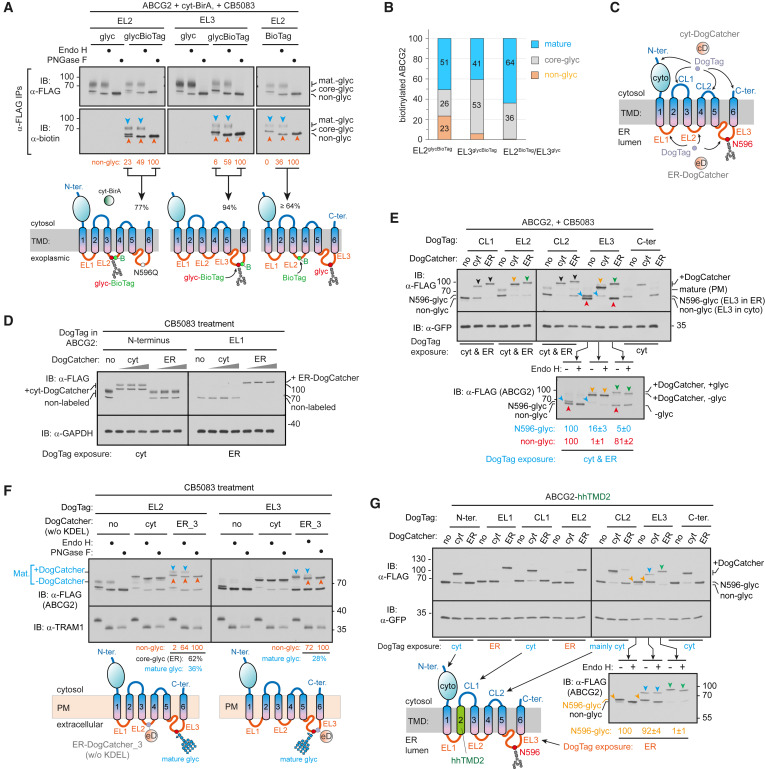
After the rearrangement of TMHs with incorrect orientation, this immature intermediate can dimerize to form a quaternary structure, which may promote the subsequent assembly of pTMH2 with other transmembrane spiral bundles, allowing pTMH2 to integrate into the membrane and form the final structure surrounded by other TMHs. This study revealed a functionally critical but lipid-repellent pTMH topology generation pathway, indicating that pTMH can guide the post-translational topological rearrangement of membrane proteins, thereby avoiding exposure to hydrophobic phospholipid environments during pTMH folding.
Related Products and Services
Transmembrane Proteins CD Antigens Targets of CAR-T Cell Therapy Specially Designed Labeled Protein for Car-T Therapy Cytokines Cancer Drug Targets Immune Checkpoint Proteins Protein Interaction Service Protein Expression and Purification Services Drug Discovery Screening Protein Pathway Profiling
Reference Ji J, Cui MK, Zou R, Wu MZ, Ge MX, Li J, Zhang ZR. An ATP13A1-assisted topogenesis pathway for folding multi-spanning membrane proteins. Mol Cell. 2024 May 16;84(10):1917-1931.e15. doi: 10.1016/j.molcel.2024.04.010. Epub 2024 May 8. PMID: 38723633.
