Uncategorized Tuesday, 2024/06/11
Cullin RING E3 ligase (CRL) has become a key regulator of disease modification pathways and therapeutic targets. CRL (CRL3) containing Cullin 3 (Cul3) is involved in regulating liver insulin and oxidative stress signaling. However, the function of CRL3 in liver pathophysiology is currently unclear.
Recently, a research report titled "Cullin 3 RING E3 ligase inactivation causes NRF2-dependent NADH reductive stress, hepatic lipodystrophy, and systemic insulin resistance" was published in the journal Proceedings of the National Academy of Sciences. Scientists from institutions such as the University of Oklahoma Center for Health Sciences revealed the ability of a special drug to prevent fat accumulation in the liver, which often occurs in obese patients and can lead to severe fatty liver disease.
Our Related Proteins
| Cat. No. | Product Name | Species | Source | Tag |
| IL31RA-732H | Active Recombinant Human IL31RA protein, His-tagged | Human | HEK293 | His |
| IL31RA-1350H | Active Recombinant Human IL31RA protein(Met1-Ser516), hFc-tagged | Human | HEK293 | C-hFc |
| IL31RA-731H | Recombinant Human IL31RA protein, hFc-tagged | Human | HEK293 | C-hFc |
| IL31RA-1318H | Recombinant Human IL31RA protein, His & T7-tagged | Human | E.coli | His/T7 |
| GABPA-554H | Recombinant Human GABPA Protein, His-tagged | Human | E.coli | His |
| GABPA-2992H | Recombinant Human GABPA Protein, His (Fc)-Avi-tagged | Human | HEK293 | His (Fc)-Avi |
| GABPA-4631H | Recombinant Human GABPA Protein, GST-tagged | Human | Wheat Germ | GST |
| CUL3-148H | Active Recombinant Human CUL3/RBX1 | Human | Insect Cell | N/A |
| CUL3-321H | Recombinant Human CUL3 protein, MYC/DDK-tagged | Human | HEK293 | Myc/DDK |
This study is based on previous research, where researchers found that a drug developed to inhibit cancerous tumors may also improve insulin sensitivity and lower blood sugar levels in the body; this drug called MLN4924 can work by preventing the degradation of a specific protein required for all cells to respond to insulin. In the article, researchers studied mice and eliminated a gene called Cul3 in their liver. When this gene is present in the body, drugs inhibit its function to prevent protein degradation. Eliminating this gene may help researchers gain a more comprehensive understanding of the events that occur when mice become obese on a high-fat diet.
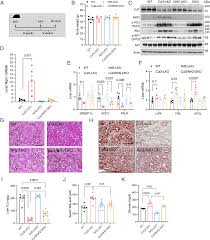
The discovery by researchers is very surprising, as without this gene, mice on a high-fat diet would not accumulate fat in their liver (despite being obese). However, the loss of fat accumulation in the liver promotes the entry of fat into the bloodstream and other tissues such as fat, which should not have stored fat. As a result, as the largest organ in the body, the muscle's response to insulin deteriorates, leading to high blood sugar in mice. Researcher Li said that by eliminating this gene, we can actively prevent the accumulation of fat in the liver, but this actually worsens insulin resistance in muscles, which may tell us that fat metabolism in these organs may be interrelated.
The relevant research results indicate that in addition to reducing fat levels in the liver, reducing obesity and improving insulin sensitivity are crucial for fatty liver disease, as these improvements outside the liver are crucial in preventing the accumulation of fat entering the liver in other tissues. The researchers also clarified why chronic diseases such as type 2 diabetes and fatty liver disease are never simple, and improvement in one research field may lead to negative effects in another field. Nevertheless, this study is very helpful for understanding the process of fatty liver disease and the role of drugs in this disease context. Researcher Friedman said that we have some promising ideas to target potential pathways to reduce fat accumulation in the liver, while also improving insulin sensitivity in the body. The drug reorientation we conducted in this study may be very exciting, as we have gained a lot of knowledge about this drug and its safety, and we believe it has great potential.
In summary, this study reveals the important role of CRL3 in the regulation mechanism of liver metabolism, and confirms that excessive activation of NRF2 downstream of CRL3 may lead to hepatic metabolic maladaptation to obesity and insulin tolerance.
Related Products and Services
CD Antigens Targets of CAR-T Cell Therapy Cytokines Cancer Drug Targets Immune Checkpoint Proteins Protein Interaction Service Protein Expression and Purification Services Drug Discovery Screening Protein Pathway Profiling
Reference Lijie Gu,Yanhong Du,Jianglei Chen, et al., Cullin 3 RING E3 ligase inactivation causes NRF2-dependent NADH reductive stress, hepatic lipodystrophy, and systemic insulin resistance, Proceedings of the National Academy of Sciences (2024). DOI:10.1073/pnas.2320934121
